This presentation contains “forward-looking statements” within the meaning of the
safe harbor provisions of the Private Securities Litigation Reform Act of 1995. Words such as “may”, “will”, “believe”, “expect”, “plan”, “anticipate” and similar expressions (as well as other words or expressions referencing future events or
circumstances) are intended to identify forward-looking statements. All statements, other than statements of historical facts, included in this presentation are forward-looking statements. These statements include, but are not limited to,
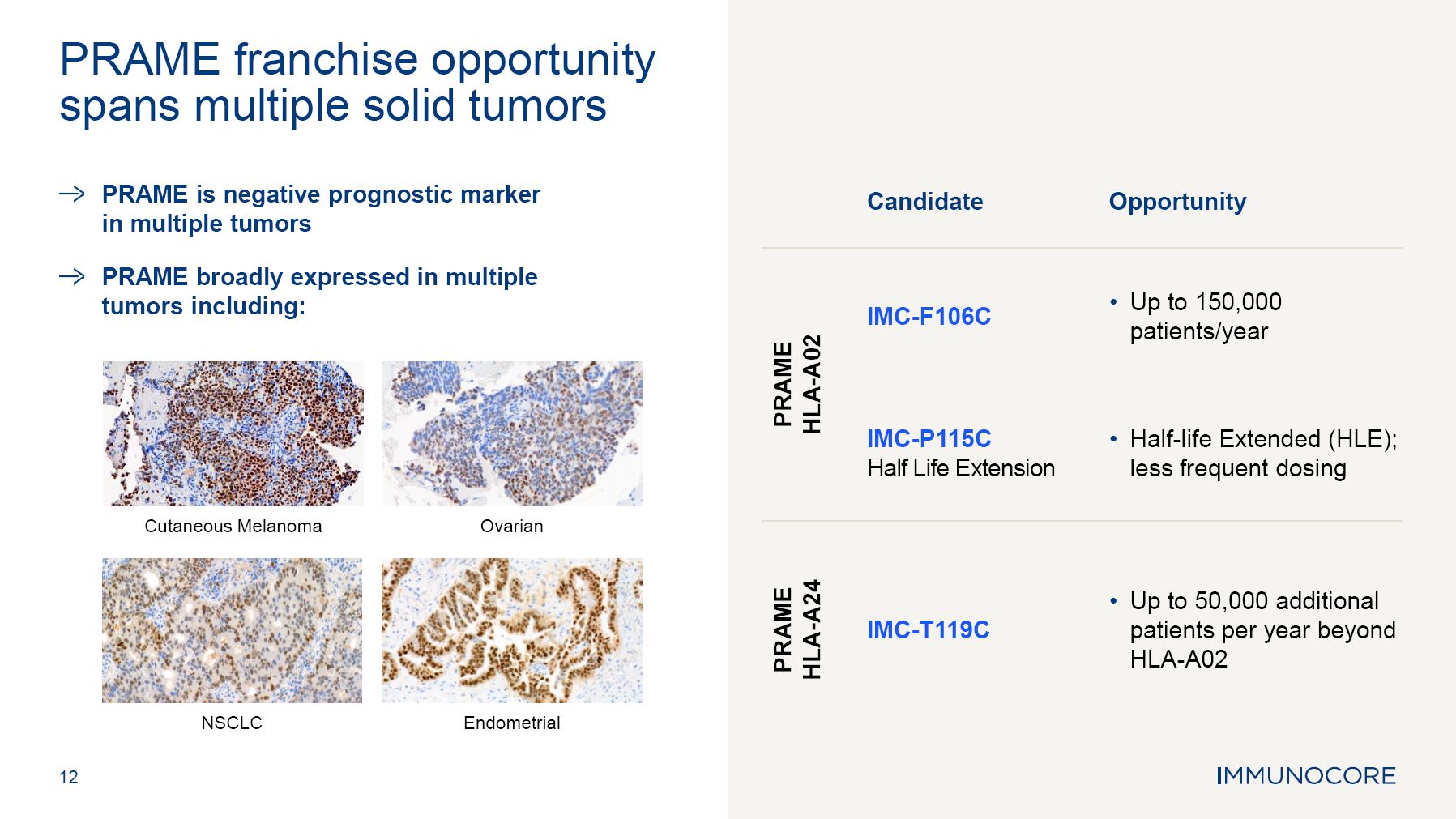
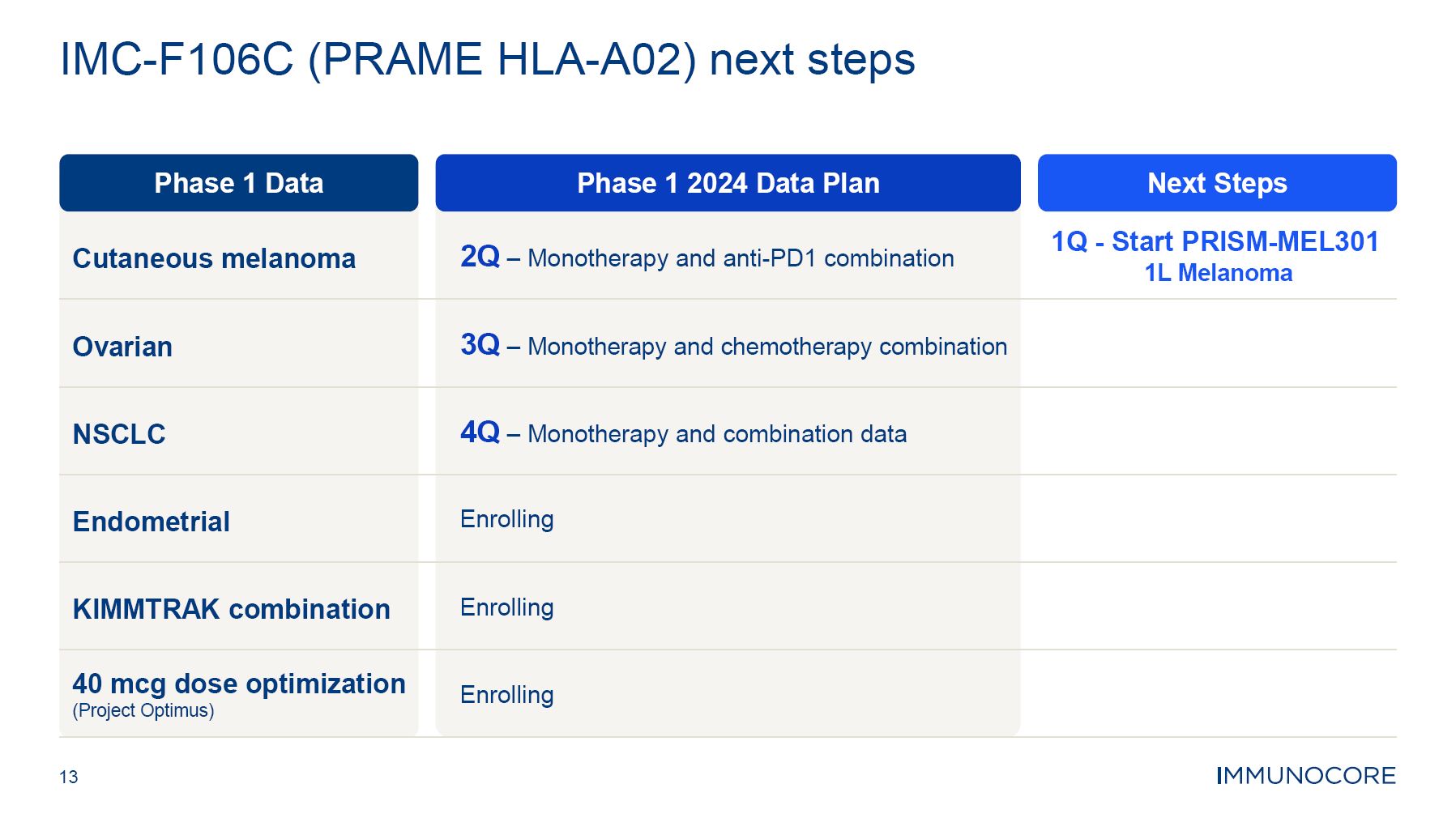
Immunocore’s capabilities across oncology, autoimmune and infectious disease therapeutic areas and its ability to grow, maximize and further develop the KIMMTRAK platform, and advance the clinical and pre-clinical programs, including the PRAME
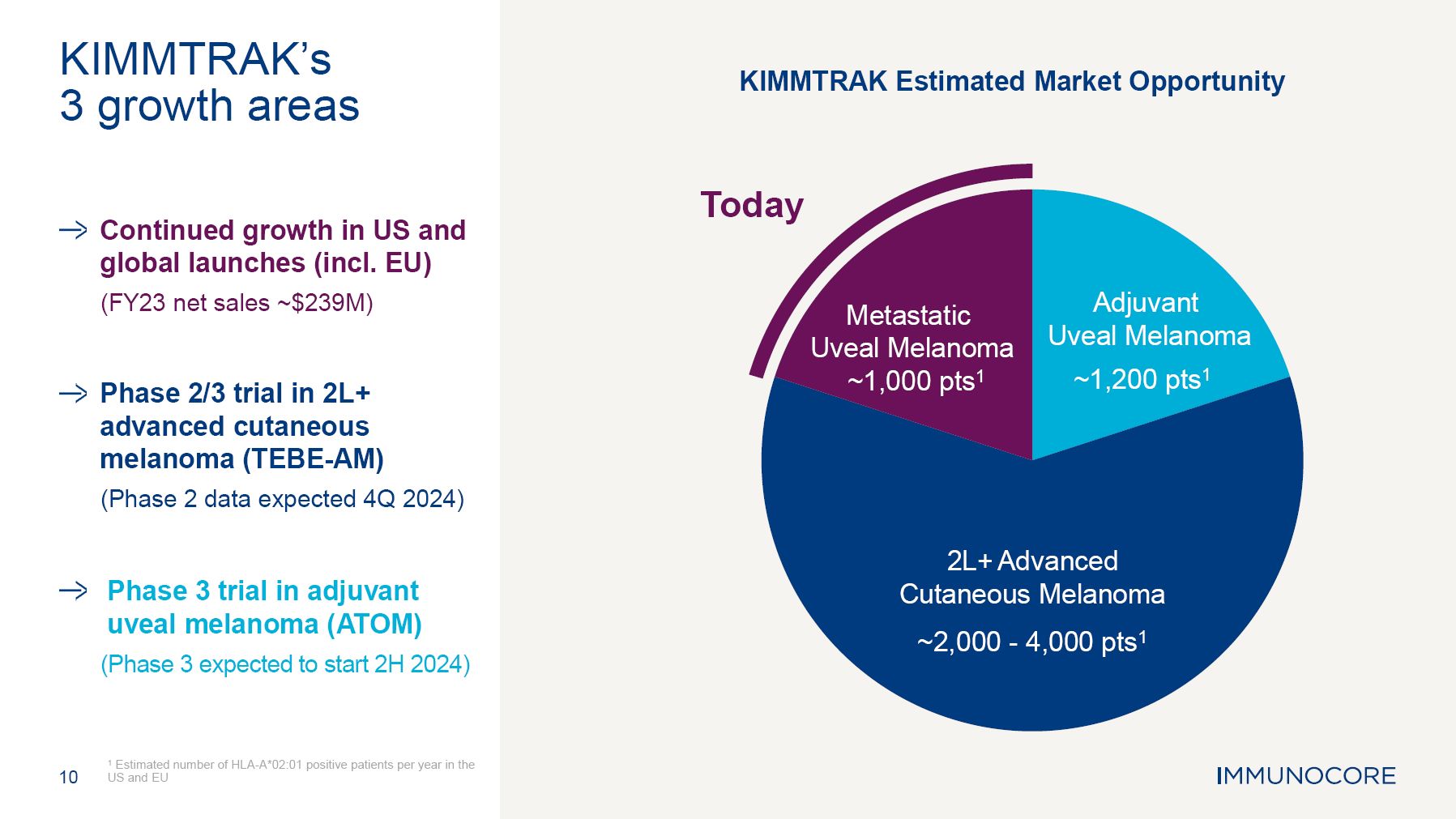
franchise, PIWIL 1 target other programs; the estimated market size and patient population for KIMMTRAK and Immunocore’s other product candidates; the three potential growth areas of KIMMTRAK including HLA-A02+ melanoma, metastatic cutaneous
melanoma and adjuvant uveal melanoma; the outlook for 2024 and growth drivers for the commercial performance of KIMMTRAK including the momentum of KIMMTRAK in the United States, planned launches in additional countries, expanded access to
KIMMTRAK in the United States and globally, early patient identification and indication expansion; expected submission of investigational new drug applications or clinical trial applications; the potential regulatory approval, expected clinical
benefits and availability of Immunocore’s product candidates; the ability to enter into pricing agreements and to translate such pricing agreement into a successful launch; the accrual assumptions regarding the outcome of price negotiations in
France; the potential benefits and advantages KIMMTRAK and Immunocore’s other product candidates will provide for patients; expectations regarding the design, progress, timing, enrollment, scope, expansion, and results of Immunocore’s existing
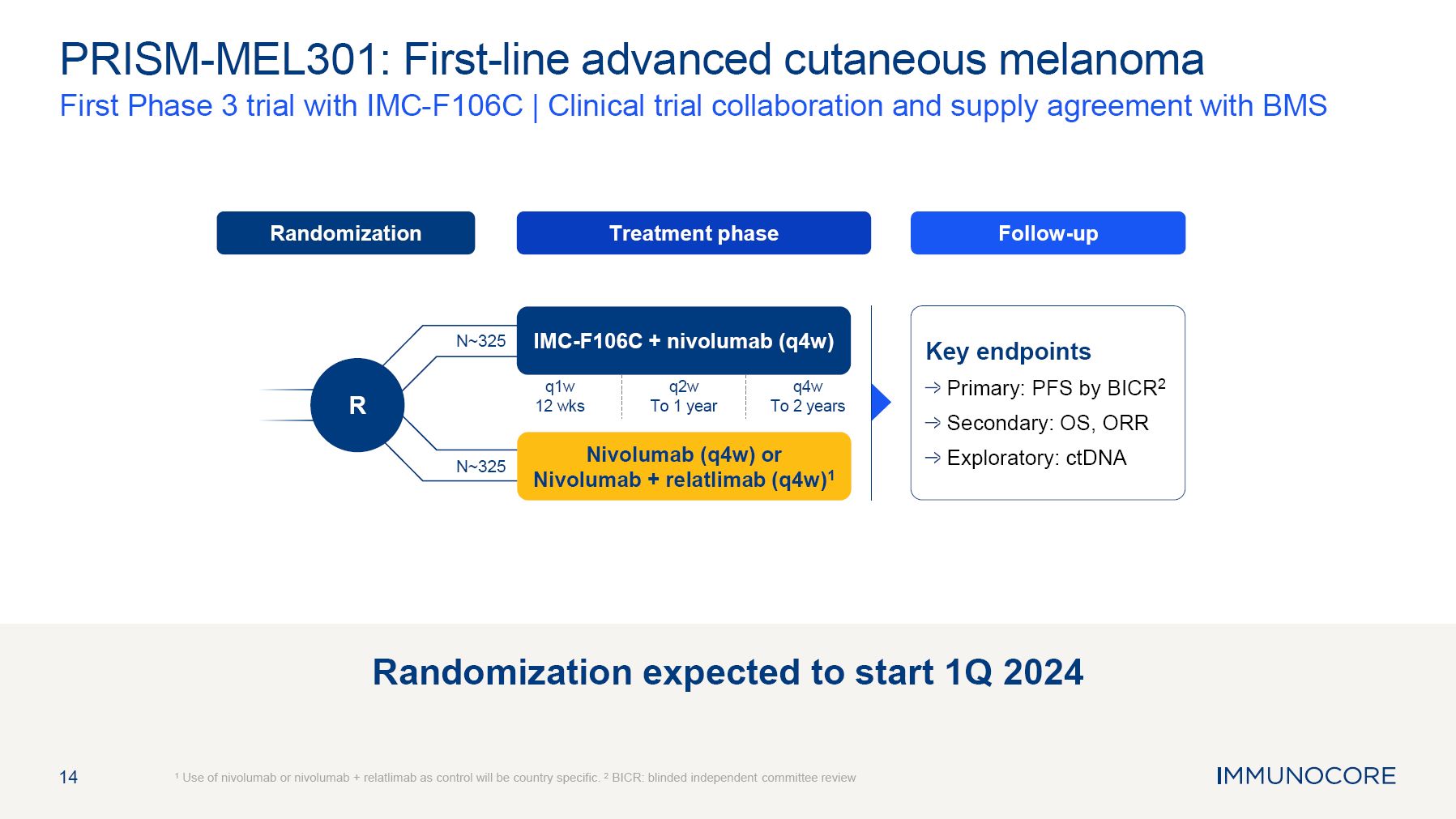
and planned clinical trials, those of Immunocore’s collaboration partners or the combined clinical trials with Immunocore’s collaboration partners; the timing and sufficiency of clinical trial outcomes to support potential approval of any of
Immunocore’s product candidates or those of, or combined with, its collaboration partners; Immunocore’s goals to develop and commercialize product candidates based on its KIMMTRAK platform alone or with collaboration partners; Immunocore’s vision
for the clinical benefit of its ImmTAAI platform; and Immunocore’s expectations regarding the use of its cash and cash equivalents, including the net proceeds from the convertible note financing. Any forward- looking statements are based on
management’s current expectations and beliefs of future events and are subject to a number of risks and uncertainties that could cause actual events or results to differ materially and adversely from those set forth in or implied by such
forward-looking statements, many of which are beyond Immunocore’s control. These risks and uncertainties include, but are not limited to, the impact of worsening macroeconomic conditions on Immunocore’s business, financial position, strategy and
anticipated milestones, including Immunocore’s ability to conduct ongoing and planned clinical trials; Immunocore’s ability to obtain a clinical supply of current or future product candidates or commercial supply of KIMMTRAK or any future
approved products, including as a result of health epidemics or pandemic, war in Ukraine, the conflict between Hamas and Israel, or global geopolitical tension; Immunocore’s ability to obtain and maintain regulatory approval of its product
candidates, including KIMMTRAK; Immunocore’s ability and plans in continuing to establish and expand a commercial infrastructure and to successfully launch, market and sell KIMMTRAK and any future approved products; Immunocore’s ability to
successfully expand the approved indications for KIMMTRAK or obtain marketing approval for KIMMTRAK in additional geographies in the future; the delay of any current or planned clinical trials, whether due to patient enrollment delays or
otherwise; Immunocore’s ability to successfully demonstrate the safety and efficacy of its product candidates and gain approval of its product candidates on a timely basis, if at all; competition with respect to market opportunities; unexpected
safety or efficacy data observed during preclinical studies or clinical trials; actions of regulatory agencies, which may affect the initiation, timing and progress of clinical trials or future regulatory approval; Immunocore’s need for and
ability to obtain additional funding, on favorable terms or at all, including as a result of worsening macroeconomic conditions, including changes inflation and interest rates and unfavorable general market conditions, and the impacts thereon of
the war in Ukraine, the conflict between Hamas and Israel, and global geopolitical tension; Immunocore’s ability to obtain, maintain and enforce intellectual property protection for KIMMTRAK or any product candidates it is developing; and the
success of Immunocore’s current and future collaborations, partnerships or licensing arrangements. These and other risks and uncertainties are described in greater detail in the section titled "Risk Factors" in Immunocore’s filings with the
Securities and Exchange Commission, including Immunocore’s most recent Annual Report on Form 10- K for the year ended December 31, 2023 filed with the Securities and Exchange Commission on February 28, 2024, as well as discussions of potential
risks, uncertainties, and other important factors in Immunocore’s subsequent filings with the Securities and Exchange Commission. All forward looking statements contained in this presentation speak only as of the date on which they were made and
should not be relied upon as representing its views as of any subsequent date. Except to the extent required by law, Immunocore undertakes no obligation to update such statements to reflect events that occur or circumstances that exist after the
date on which they were made. Certain information contained in this presentation relates to or is based on studies, publications, surveys, and other data obtained from third party sources and Immunocore’s own internal estimates and research.
While Immunocore believes these third party sources to be reliable as of the date of this presentation, it has not independently verified, and makes no representation as to the adequacy, fairness, accuracy, or completeness of, any information
obtained from third party sources. KIMMTRAK™ is a trademark owned or licensed to Immunocore. 2 Forward Looking Statements