Exhibit 99.3
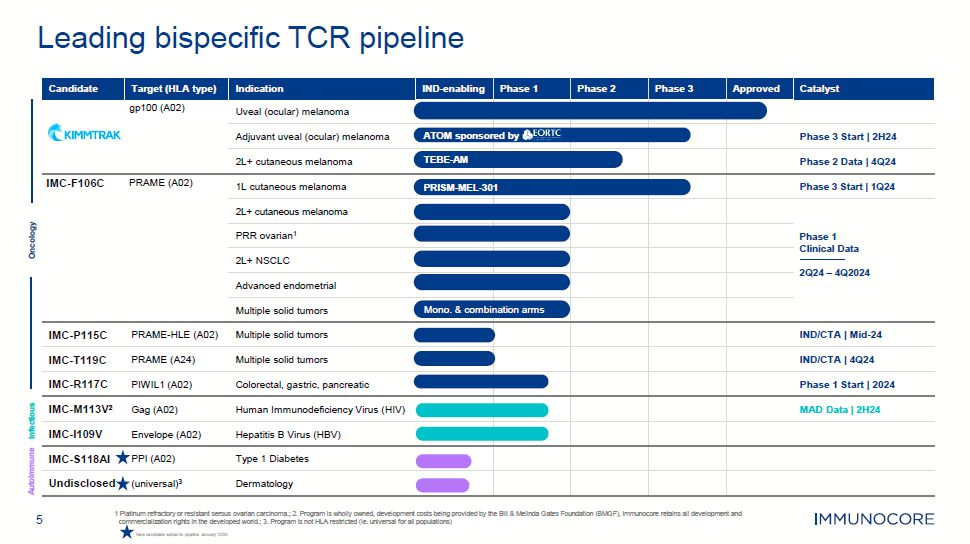
5 1 Platinum refractory or resistant serous ovarian carcinoma.; 2. Program is
wholly owned, development costs being provided by the Bill & Melinda Gates Foundation (BMGF), Immunocore retains all development and commercialization rights in the developed world.; 3. Program is not HLA restricted (ie. universal for all
populations) Leading bispecific TCR pipeline Oncology Infectious Autoimmune Candidate Target (HLA type) gp100 (A02) Indication Uveal (ocular) melanoma IND-enabling Phase 1 Phase 2 Phase 3 Approved Catalyst Adjuvant uveal
(ocular) melanoma ATOM sponsore d by Phase 3 Start | 2H24 2L+ cutaneous melanoma TEBE-AM Phase 2 Data | 4Q24 IMC-F106C PRAME (A02) 1L cutaneous melanoma PRISM-MEL-301 Phase 3 Start | 1Q24 2L+ cutaneous melanoma Phase 1 Clinical
Data 2Q24 – 4Q2024 PRR ovarian1 2L+ NSCLC Advanced endometrial Multiple solid tumors Mono. & combina tion arms IMC-P115C PRAME-HLE (A02) Multiple solid tumors IND/CTA | Mid-24 IMC-T119C PRAME (A24) Multiple solid
tumors IND/CTA | 4Q24 IMC-R117C PIWIL1 (A02) Colorectal, gastric, pancreatic Phase 1 Start | 2024 IMC-M113V2 Gag (A02) Human Immunodeficiency Virus (HIV) MAD Data | 2H24 IMC-I109V Envelope (A02) Hepatitis B Virus
(HBV) IMC-S118AI PPI (A02) Type 1 Diabetes Undisclosed (universal)3 Dermatology New candidate added to pipeline January 2024.