Exhibit 99.2

Transformative immunomodulating medicines for patients January 2024

This presentation contains “forward-looking statements” within the meaning of
the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. Words such as “may”, “will”, “believe”, “expect”, “plan”, “anticipate” and similar expressions (as well as other words or expressions referencing future
events or circumstances) are intended to identify forward-looking statements. All statements, other than statements of historical facts, included in this presentation are forward-looking statements. These statements include, but are not
limited to, Immunocore’s capabilities across oncology, autoimmune and infectious disease therapeutic areas and its ability to grow and further development the PRAME franchise; the estimated market size and patient population for KIMMTRAK and
Immunocore’s other product candidates; the three growth areas of KIMMTRAK including HLA-A02+ melanoma, metastatic cutaneous melanoma and adjuvant uveal melanoma; expected submission of investigational new drug applications or clinical trial
applications; the potential regulatory approval, expected clinical benefits and availability of Immunocore’s product candidates; the commercial performance of KIMMTRAK including planned launches in additional countries, expanded access to
KIMMTRAK in the United States and globally, and indication expansion; the ability to enter into pricing agreements and to translate such pricing agreement into a successful launch; the potential benefits and advantages KIMMTRAK and
Immunocore’s other product candidates will provide for patients; the benefits of Immunocore’s collaboration with the European Organisation for Research and Treatment of Cancer (EORTC); expectations regarding the design, progress, timing,
enrollment, scope, expansion, and results of Immunocore’s existing and planned clinical trials, those of Immunocore’s collaboration partners or the combined clinical trials with Immunocore’s collaboration partners; the timing and sufficiency
of clinical trial outcomes to support potential approval of any of Immunocore’s product candidates or those of, or combined with, its collaboration partners; Immunocore’s goals to develop and commercialize product candidates based on its
KIMMTRAK platform alone or with collaboration partners; Immunocore’s ability to develop new product candidates using its discovery engine; Immunocore’s ability to initiate CMC manufacturing for autoimmune candidates on the expected timeline,
or at all; potential growth opportunities and trends, including in connection with product launches; and Immunocore’s preliminary unaudited cash and cash equivalents. Any forward-looking statements are based on management’s current
expectations and beliefs of future events and are subject to a number of risks and uncertainties that could cause actual events or results to differ materially and adversely from those set forth in or implied by such forward-looking
statements, many of which are beyond Immunocore’s control. These risks and uncertainties include, but are not limited to, the impact of worsening macroeconomic conditions on Immunocore’s business, financial position, strategy and anticipated
milestones, including Immunocore’s ability to conduct ongoing and planned clinical trials; Immunocore’s ability to obtain a clinical supply of curr ent or future product candidates or commercial supply of KIMMTRAK or any future approved
products, including as a result of health epidemics or pandemic, war in Ukraine, the conflict between Hamas and Israel, or global geopolitical tension; Immunocore’s ability to obtain and maintain regulatory approval of its product candidates,
including KIMMTRAK; Immunocore’s ability and plans in continuing to establish and expand a commercial infrastructure and to successfully launch, market and sell KIMMTRAK and any future approved products; Immunocore’s ability to successfully
expand the approved indications for KIMMTRAK or obtain marketing approval for KIMMTRAK in additional geographies in the future; the delay of any current or planned clinical trials, whether due to patient enrollment delays or otherwise;
Immunocore’s ability to successfully demonstrate the safety and efficacy of its product candidates and gain approval of its product candidates on a timely basis, if at all; competition with respect to market opportunities; unexpected safety
or efficacy data observed during preclinical studies or clinical trials; actions of regulatory agencies, which may affect the initiation, timing and progress of clinical trials or future regulatory approval; Immunocore’s need for and ability
to obtain additional funding, on favorable terms or at all, including as a result of worsening macroeconomic conditions, including changes inflation and interest rates and unfavorable general market conditions, and the impacts thereon of the
war in Ukraine, the conflict between Hamas and Israel, and global geopolitical tension; Immunocore’s ability to obtain, maintain and enforce intellectual property protection for KIMMTRAK or any product candidates it is developing; and the
success of Immunocore’s current and future collaborations, partnerships or licensing arrangements. These and other risks and uncertainties are described in greater detail in the section titled "Risk Factors" in Immunocore’s filings with the
Securities and Exchange Commission, including Immunocore’s most recent Annual Report on Form 20-F for the year ended December 31, 2022 filed with the Securities and Exchange Commission on March 1, 2023, as well as discussions of potential
risks, uncertainties, and other important factors in Immunocore’s subsequent filings with the Securities and Exchange Commission. All forward looking statements contained in this presentation speak only as of the date on which they were made
and should not be relied upon as representing its views as of any subsequent date. Except to the extent required by law, Immunocore undertakes no obligation to update such statements to reflect events that occur or circumstances that exist
after the date on which they were made. Certain information contained in this presentation relates to or is based on studies, publications, surveys, and other data obtained from third party sources and Immunocore’s own internal estimates and
research. While Immunocore believes these third party sources to be reliable as of the date of this presentation, it has not independently verified, and makes no representation as to the adequacy, fairness, accuracy, or completeness of, any
information obtained from third party sources. KIMMTRAK™ is a trademark owned or licensed to Immunocore. 2 Forward Looking Statements
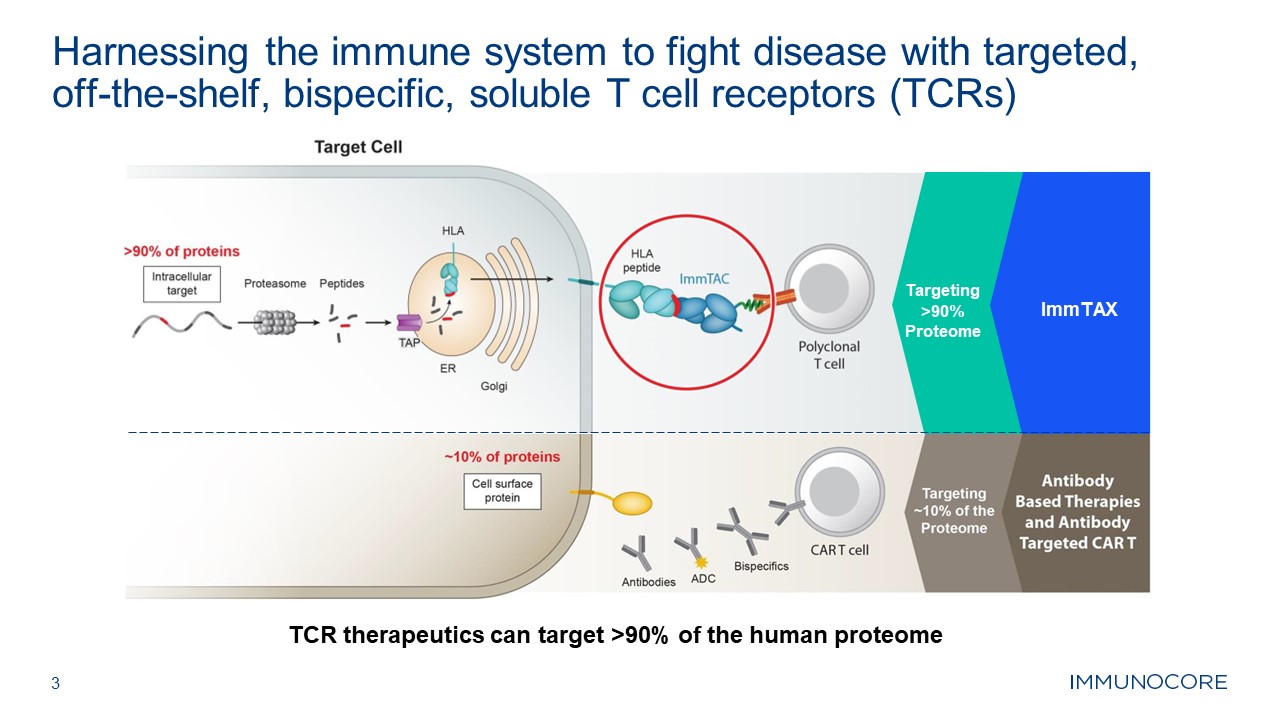
3 Harnessing the immune system to fight disease with targeted, off-the-shelf,
bispecific, soluble T cell receptors (TCRs) TCR therapeutics can target >90% of the human proteome Targeting >90% Proteome ImmTAX
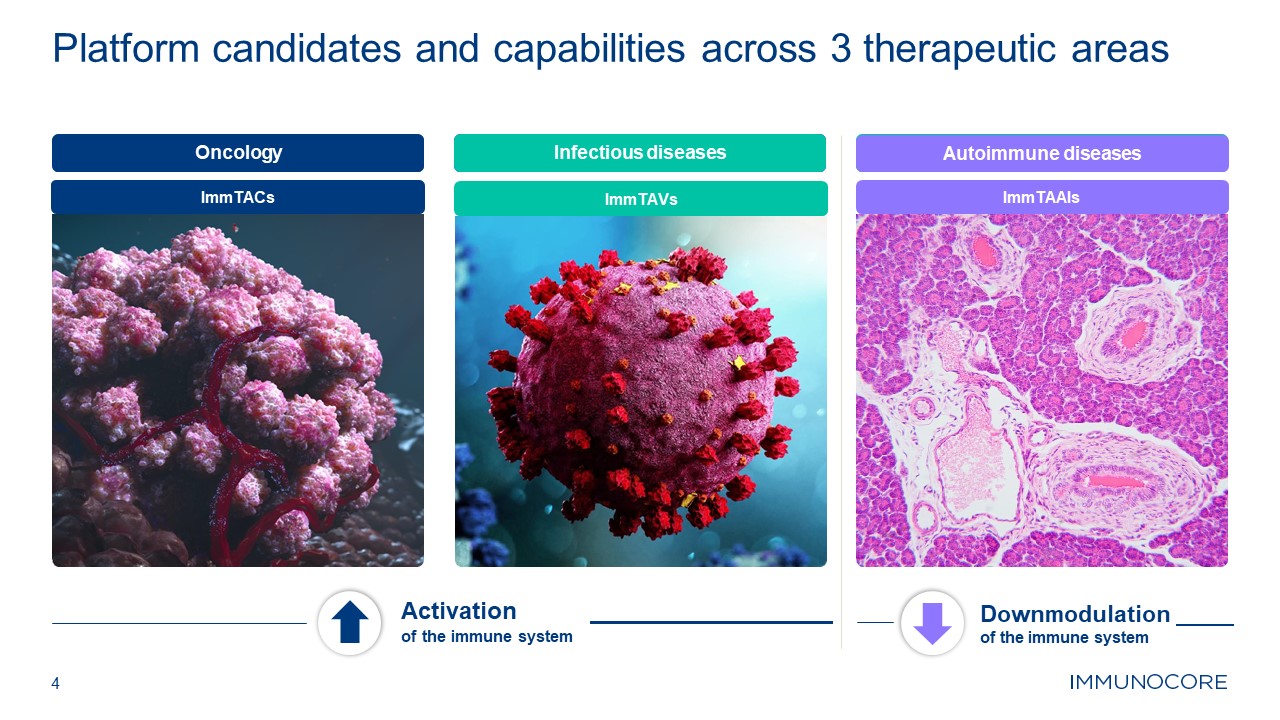
4 Platform candidates and capabilities across 3 therapeutic
areas Oncology Activation of the immune system Downmodulation of the immune system Infectious diseases Autoimmune diseases ImmTACs ImmTAVs ImmTAAIs
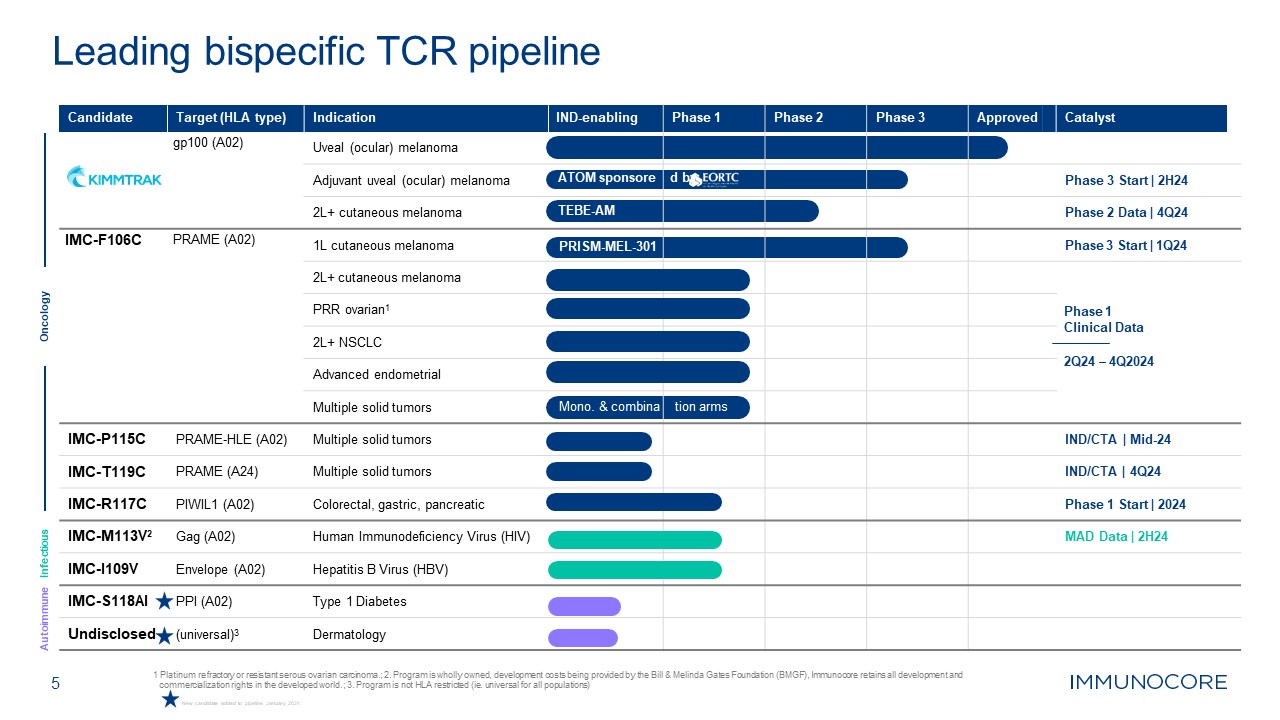
5 1 Platinum refractory or resistant serous ovarian carcinoma.; 2. Program is
wholly owned, development costs being provided by the Bill & Melinda Gates Foundation (BMGF), Immunocore retains all development and commercialization rights in the developed world.; 3. Program is not HLA restricted (ie. universal for all
populations) Leading bispecific TCR pipeline Oncology Infectious Autoimmune Candidate Target (HLA type) gp100 (A02) Indication Uveal (ocular) melanoma IND-enabling Phase 1 Phase 2 Phase 3 Approved Catalyst Adjuvant uveal
(ocular) melanoma ATOM sponsore d by Phase 3 Start | 2H24 2L+ cutaneous melanoma TEBE-AM Phase 2 Data | 4Q24 IMC-F106C PRAME (A02) 1L cutaneous melanoma PRISM-MEL-301 Phase 3 Start | 1Q24 2L+ cutaneous melanoma Phase 1 Clinical
Data 2Q24 – 4Q2024 PRR ovarian1 2L+ NSCLC Advanced endometrial Multiple solid tumors Mono. & combina tion arms IMC-P115C PRAME-HLE (A02) Multiple solid tumors IND/CTA | Mid-24 IMC-T119C PRAME (A24) Multiple solid
tumors IND/CTA | 4Q24 IMC-R117C PIWIL1 (A02) Colorectal, gastric, pancreatic Phase 1 Start | 2024 IMC-M113V2 Gag (A02) Human Immunodeficiency Virus (HIV) MAD Data | 2H24 IMC-I109V Envelope (A02) Hepatitis B Virus
(HBV) IMC-S118AI PPI (A02) Type 1 Diabetes Undisclosed (universal)3 Dermatology New candidate added to pipeline January 2024.

Maximizing potential of KIMMTRAK® in HLA-A02+ melanoma 6
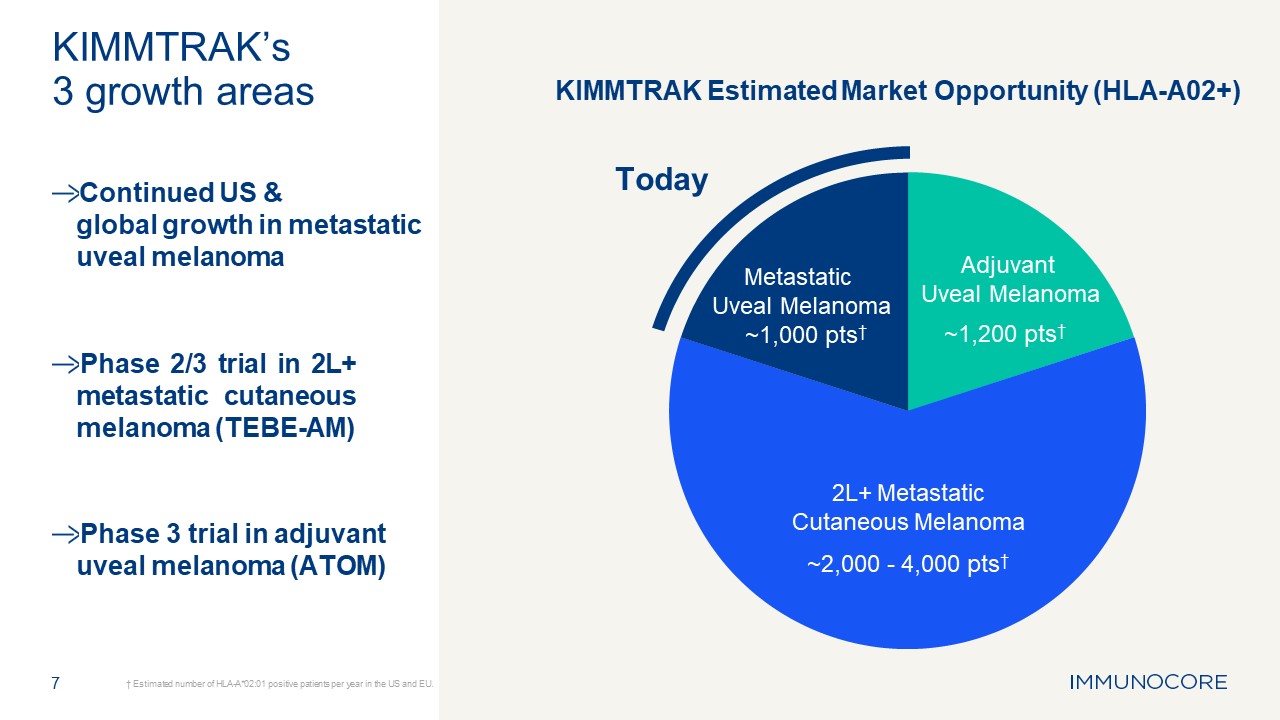
7 † Estimated number of HLA-A*02:01 positive patients per year in the US and
EU. KIMMTRAK’s 3 growth areas Continued US & global growth in metastatic uveal melanoma Phase 2/3 trial in 2L+ metastatic cutaneous melanoma (TEBE-AM) Phase 3 trial in adjuvant uveal melanoma (ATOM) Today Adjuvant Uveal
Melanoma ~1,200 pts† 2L+ Metastatic Cutaneous Melanoma ~2,000 - 4,000 pts† Metastatic Uveal Melanoma ~1,000 pts† KIMMTRAK Estimated Market Opportunity (HLA-A02+)
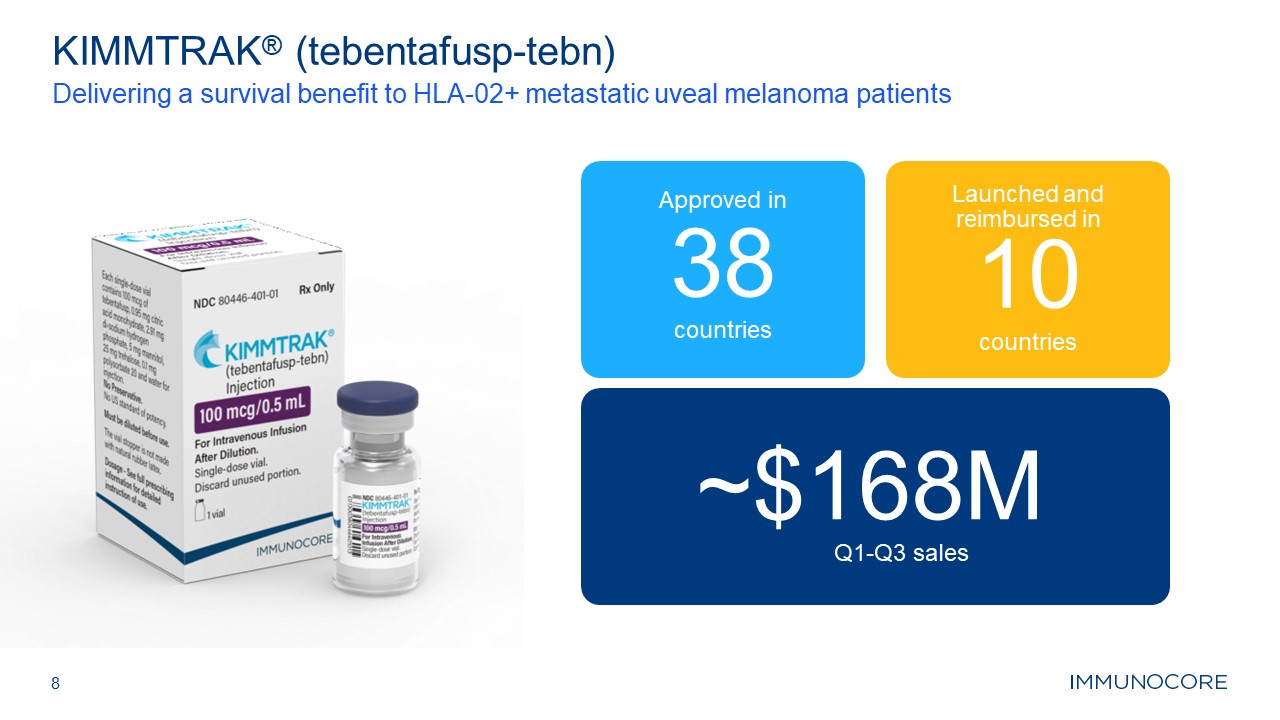
8 KIMMTRAK® (tebentafusp-tebn) Delivering a survival benefit to HLA-02+
metastatic uveal melanoma patients Approved in 38 countries Launched and reimbursed in 10 countries ~$168M Q1-Q3 sales
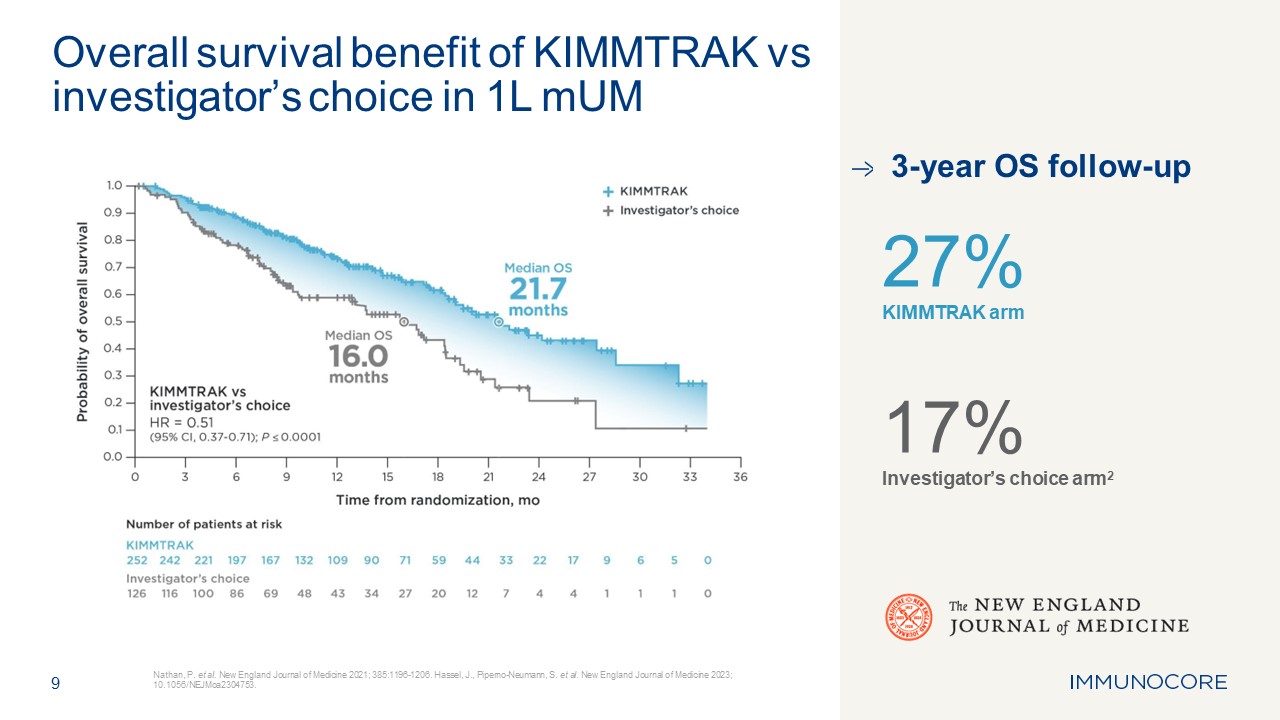
9 Nathan, P. et al. New England Journal of Medicine 2021; 385:1196-1206.
Hassel, J., Piperno-Neumann, S. et al. New England Journal of Medicine 2023; 10.1056/NEJMoa2304753. Overall survival benefit of KIMMTRAK vs investigator’s choice in 1L mUM 3-year OS follow-up 27% KIMMTRAK arm 17% Investigator’s choice
arm2
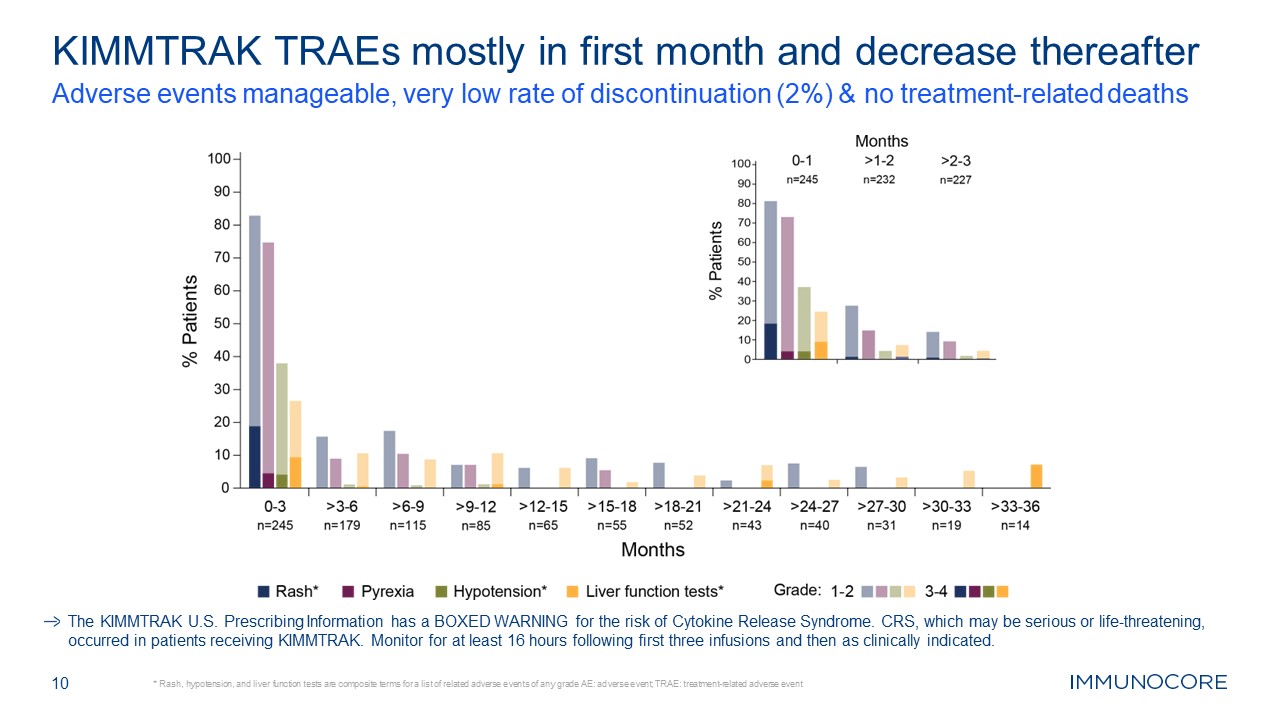
10 KIMMTRAK TRAEs mostly in first month and decrease thereafter Adverse events
manageable, very low rate of discontinuation (2%) & no treatment-related deaths * Rash, hypotension, and liver function tests are composite terms for a list of related adverse events of any grade AE: adverse event; TRAE:
treatment-related adverse event The KIMMTRAK U.S. Prescribing Information has a BOXED WARNING for the risk of Cytokine Release Syndrome. CRS, which may be serious or life-threatening, occurred in patients receiving KIMMTRAK. Monitor for at
least 16 hours following first three infusions and then as clinically indicated.
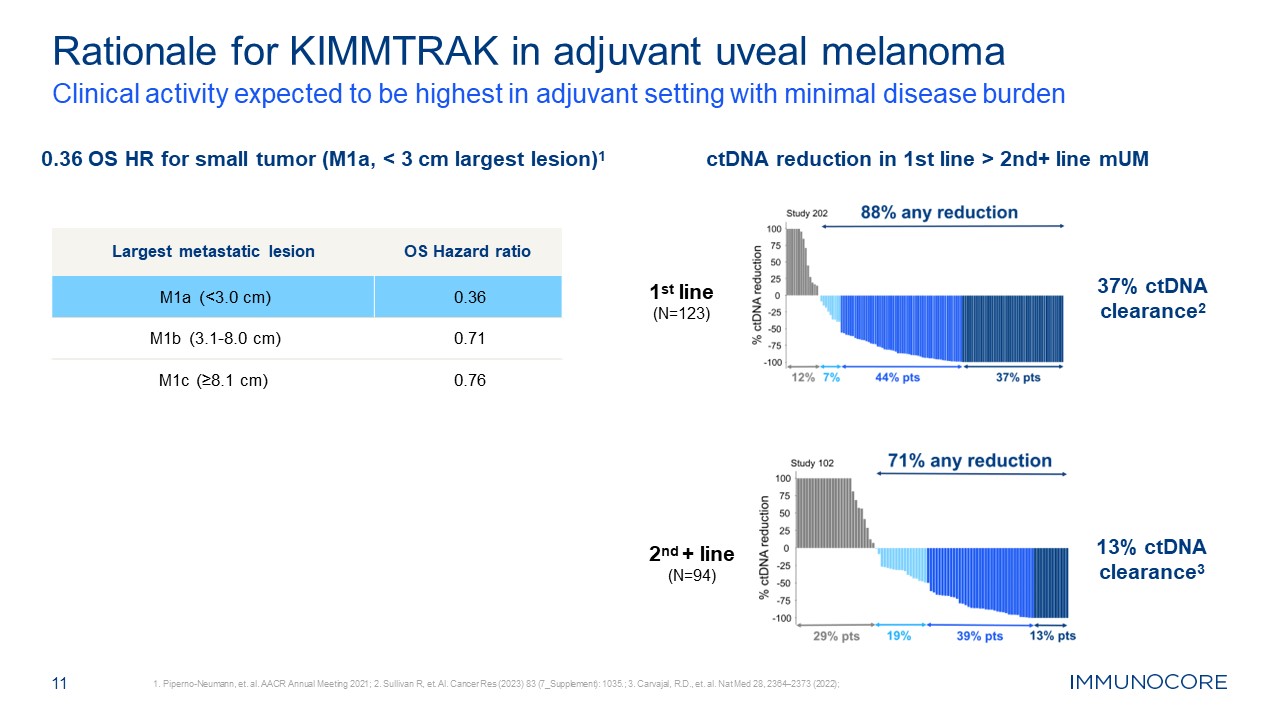
Rationale for KIMMTRAK in adjuvant uveal melanoma Clinical activity expected to
be highest in adjuvant setting with minimal disease burden 1. Piperno-Neumann, et. al. AACR Annual Meeting 2021; 2. Sullivan R, et. Al. Cancer Res (2023) 83 (7_Supplement): 1035.; 3. Carvajal, R.D., et. al. Nat Med 28, 2364–2373
(2022); 11 0.36 OS HR for small tumor (M1a, < 3 cm largest lesion)1 ctDNA reduction in 1st line > 2nd+ line mUM 1st line (N=123) 37% ctDNA clearance2 2nd + line (N=94) 13% ctDNA clearance3 Largest metastatic lesion OS Hazard
ratio M1a (<3.0 cm) 0.36 M1b (3.1-8.0 cm) 0.71 M1c (≥8.1 cm) 0.76
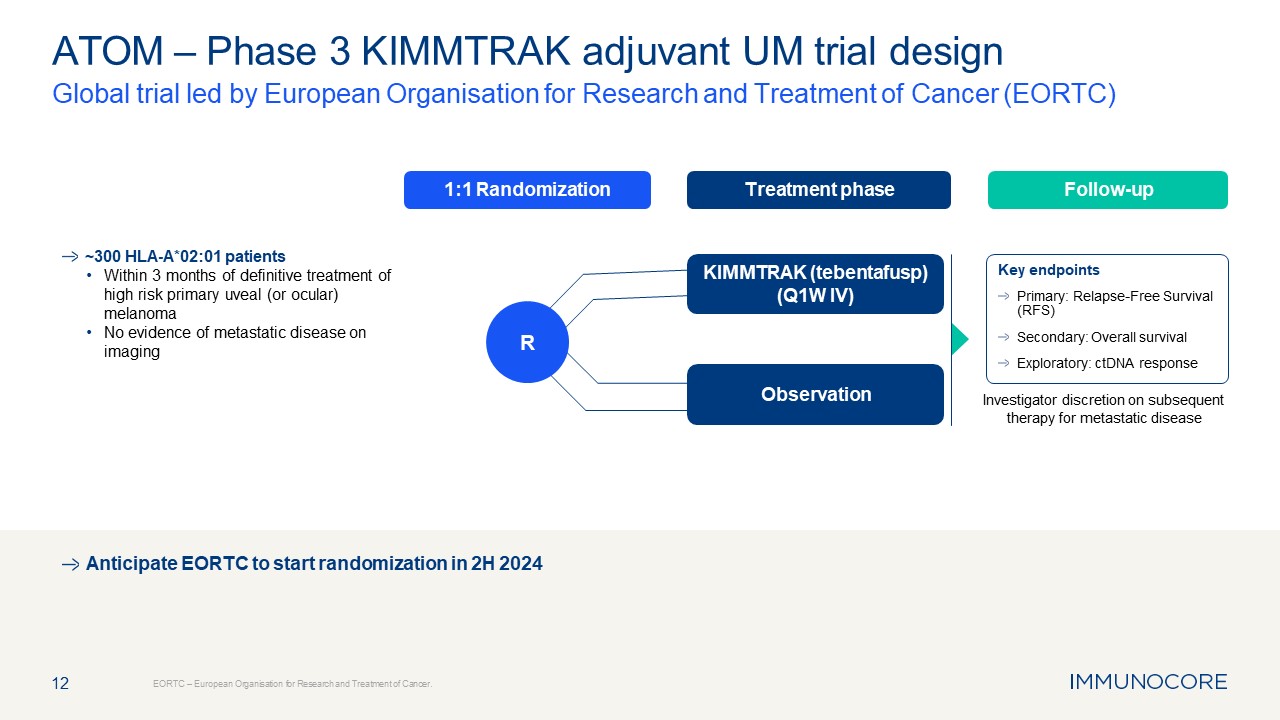
Treatment phase Follow-up 1:1 Randomization 12 ATOM – Phase 3 KIMMTRAK
adjuvant UM trial design Global trial led by European Organisation for Research and Treatment of Cancer (EORTC) EORTC – European Organisation for Research and Treatment of Cancer. Investigator discretion on subsequent therapy for
metastatic disease Anticipate EORTC to start randomization in 2H 2024 KIMMTRAK (tebentafusp) (Q1W IV) R ~300 HLA-A*02:01 patients Within 3 months of definitive treatment of high risk primary uveal (or ocular) melanoma No evidence of
metastatic disease on imaging Key endpoints Primary: Relapse-Free Survival (RFS) Secondary: Overall survival Exploratory: ctDNA response Observation
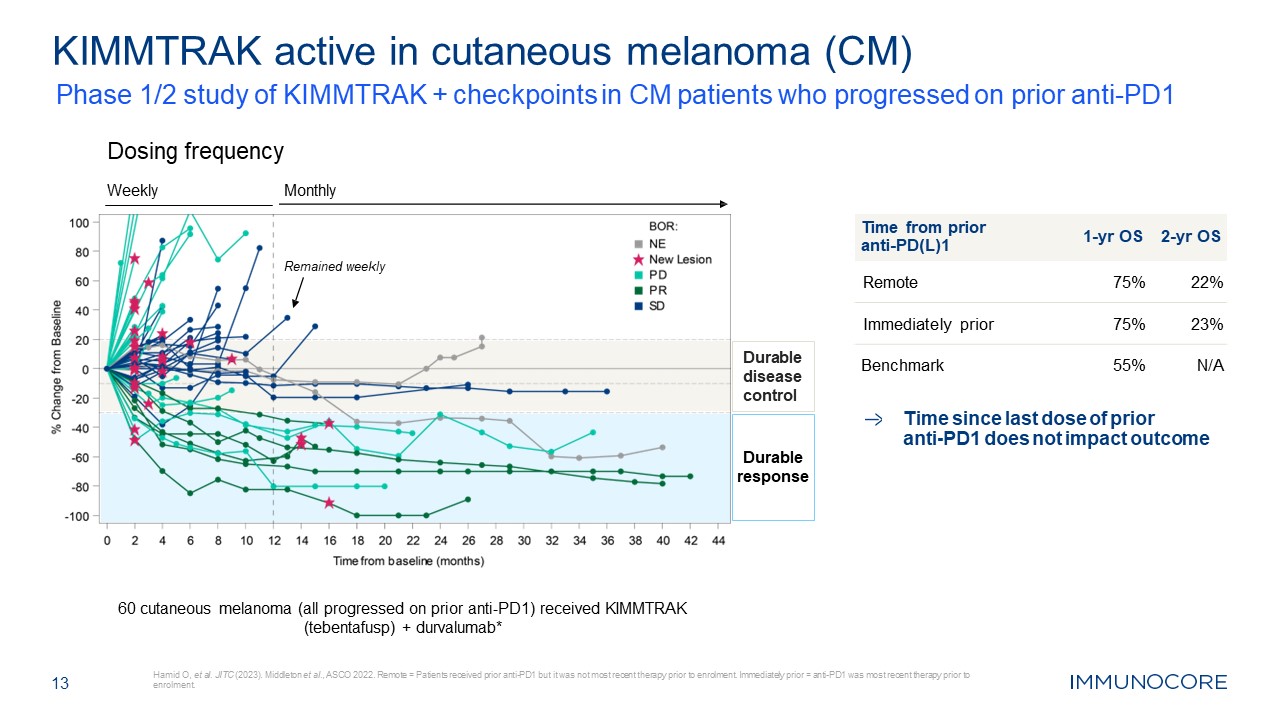
13 Hamid O, et al. JITC (2023). Middleton et al., ASCO 2022. Remote = Patients
received prior anti-PD1 but it was not most recent therapy prior to enrolment. Immediately prior = anti-PD1 was most recent therapy prior to enrolment. KIMMTRAK active in cutaneous melanoma (CM) Time from prior anti-PD(L)1 1-yr OS 2-yr
OS Remote 75% 22% Immediately prior 75% 23% Benchmark 55% N/A Monthly Dosing frequency Weekly Remained weekly Durable disease control 60 cutaneous melanoma (all progressed on prior anti-PD1) received KIMMTRAK (tebentafusp) +
durvalumab* Time since last dose of prior anti-PD1 does not impact outcome Durable response Phase 1/2 study of KIMMTRAK + checkpoints in CM patients who progressed on prior anti-PD1
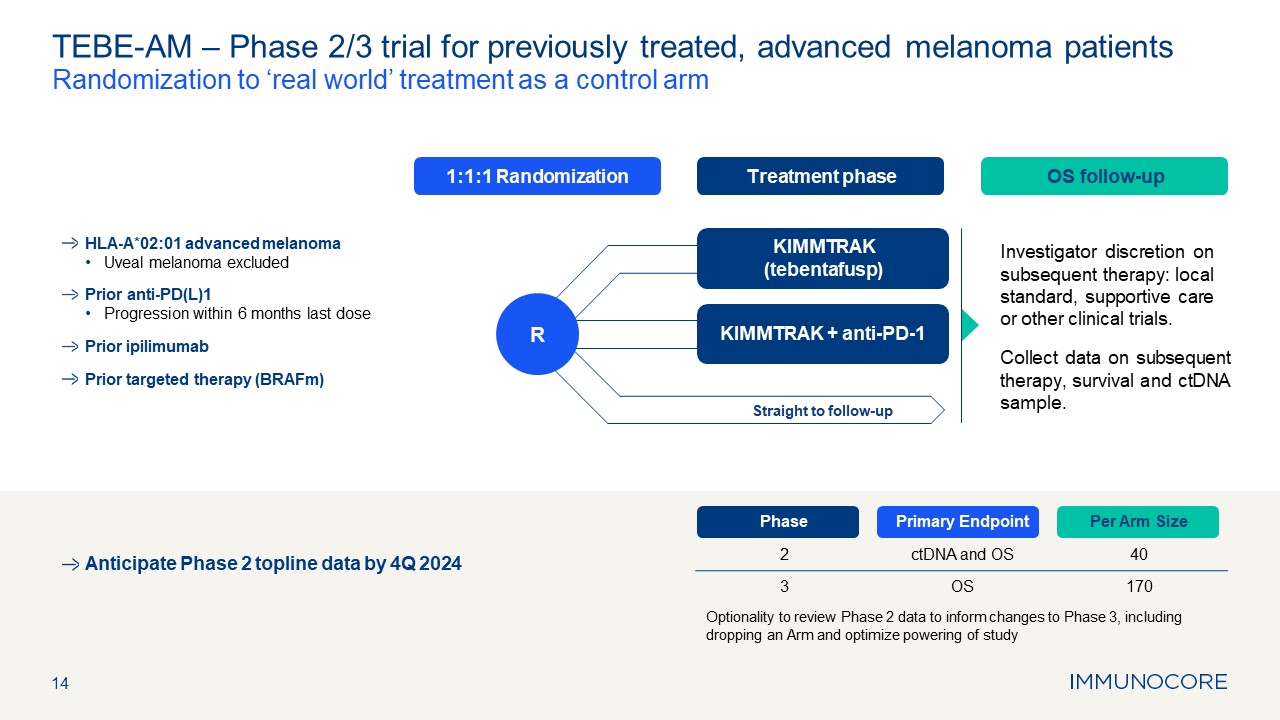
Treatment phase OS follow-up 1:1:1 Randomization 14 TEBE-AM – Phase 2/3
trial for previously treated, advanced melanoma patients Randomization to ‘real world’ treatment as a control arm Investigator discretion on subsequent therapy: local standard, supportive care or other clinical trials. Collect data on
subsequent therapy, survival and ctDNA sample. Phase Primary Endpoint Per Arm Size 2 ctDNA and OS 40 3 OS 170 Optionality to review Phase 2 data to inform changes to Phase 3, including dropping an Arm and optimize powering of
study KIMMTRAK (tebentafusp) KIMMTRAK + anti-PD-1 Straight to follow-up R HLA-A*02:01 advanced melanoma Uveal melanoma excluded Prior anti-PD(L)1 Progression within 6 months last dose Prior ipilimumab Prior targeted therapy
(BRAFm) Anticipate Phase 2 topline data by 4Q 2024

PRAME Franchise: A02, A24, A02-HLE 15
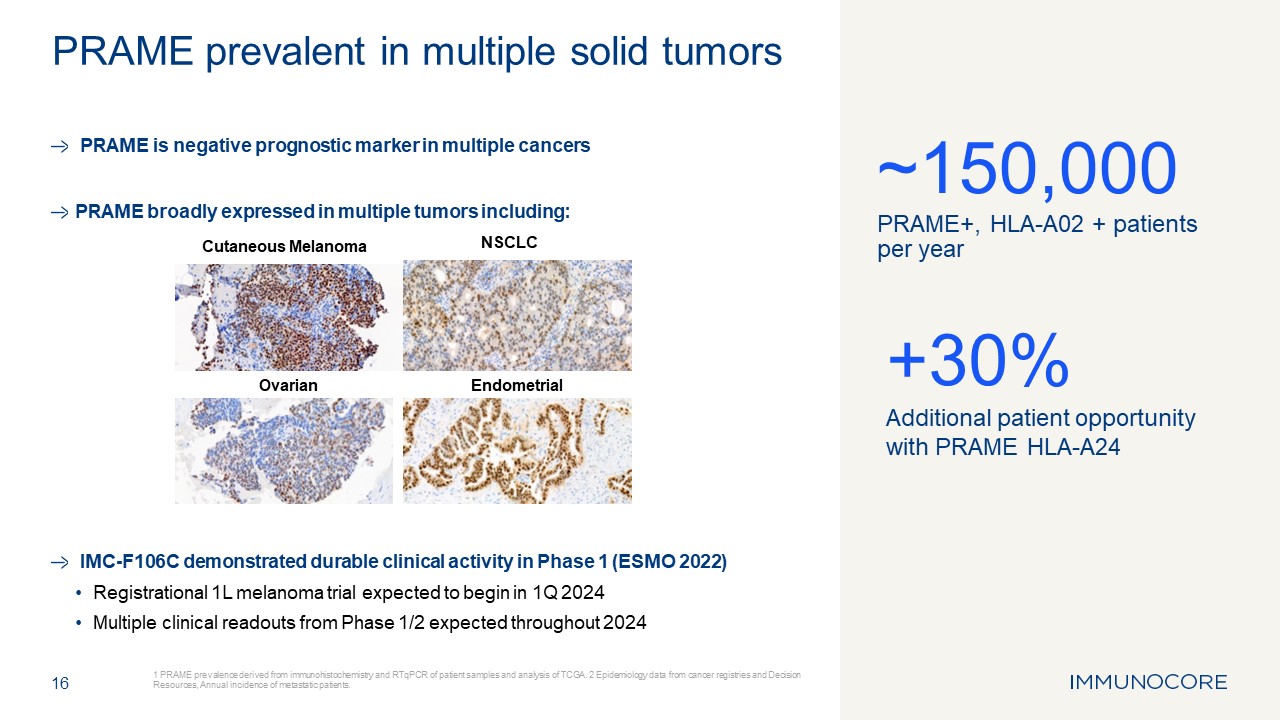
PRAME is negative prognostic marker in multiple cancers PRAME broadly expressed
in multiple tumors including: IMC-F106C demonstrated durable clinical activity in Phase 1 (ESMO 2022) Registrational 1L melanoma trial expected to begin in 1Q 2024 Multiple clinical readouts from Phase 1/2 expected throughout 2024 16 1
PRAME prevalence derived from immunohistochemistry and RTqPCR of patient samples and analysis of TCGA. 2 Epidemiology data from cancer registries and Decision Resources, Annual incidence of metastatic patients. PRAME prevalent in multiple
solid tumors ~150,000 PRAME+, HLA-A02 + patients per year +30% Additional patient opportunity with PRAME HLA-A24 Cutaneous Melanoma NSCLC Ovarian Endometrial
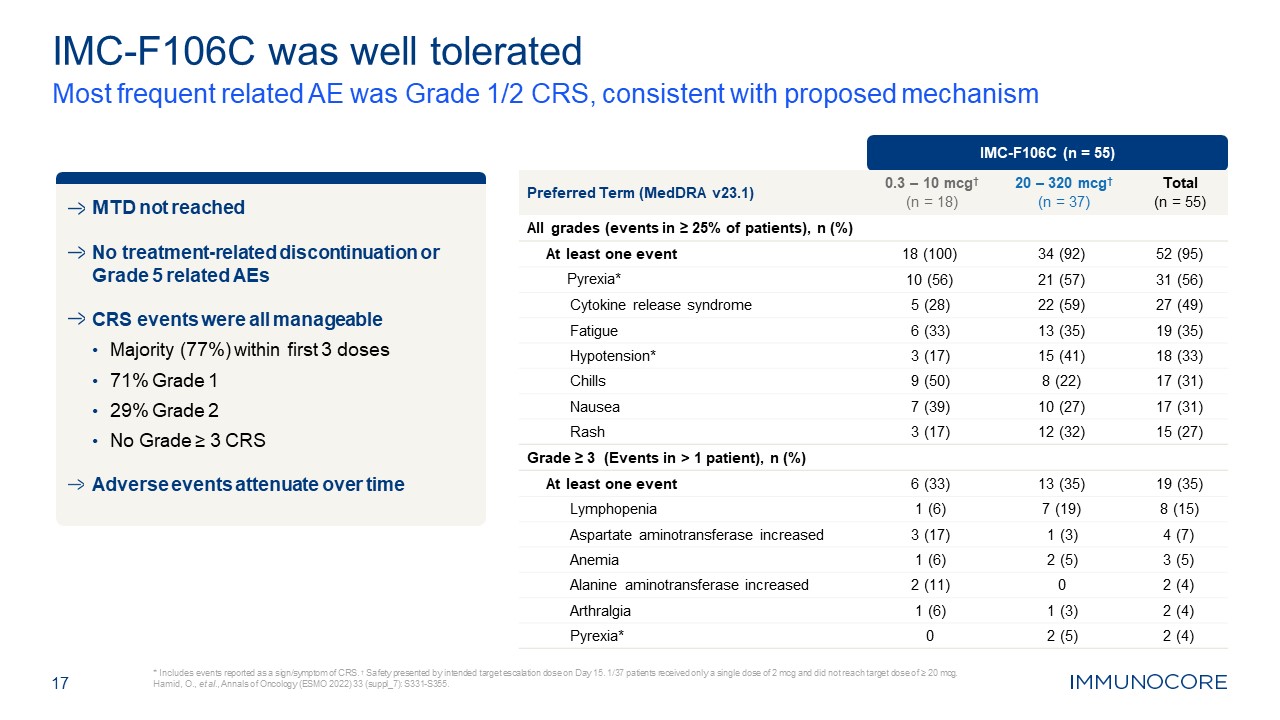
IMC-F106C (n = 55) Preferred Term (MedDRA v23.1) 0.3 – 10 mcg† (n = 18) 20 –
320 mcg† (n = 37) Total (n = 55) All grades (events in ≥ 25% of patients), n (%) At least one event 18 (100) 34 (92) 52 (95) Pyrexia* 10 (56) 21 (57) 31 (56) Cytokine release syndrome 5 (28) 22 (59) 27 (49) Fatigue 6
(33) 13 (35) 19 (35) Hypotension* 3 (17) 15 (41) 18 (33) Chills 9 (50) 8 (22) 17 (31) Nausea 7 (39) 10 (27) 17 (31) Rash 3 (17) 12 (32) 15 (27) Grade ≥ 3 (Events in > 1 patient), n (%) At least one event 6 (33) 13
(35) 19 (35) Lymphopenia 1 (6) 7 (19) 8 (15) Aspartate aminotransferase increased 3 (17) 1 (3) 4 (7) Anemia 1 (6) 2 (5) 3 (5) Alanine aminotransferase increased 2 (11) 0 2 (4) Arthralgia 1 (6) 1 (3) 2 (4) Pyrexia* 0 2
(5) 2 (4) IMC-F106C was well tolerated Most frequent related AE was Grade 1/2 CRS, consistent with proposed mechanism * Includes events reported as a sign/symptom of CRS. † Safety presented by intended target escalation dose on Day 15.
1/37 patients received only a single dose of 2 mcg and did not reach target dose of ≥ 20 mcg. Hamid, O., et al., Annals of Oncology (ESMO 2022) 33 (suppl_7): S331-S355. 17 MTD not reached No treatment-related discontinuation or Grade 5
related AEs CRS events were all manageable Majority (77%) within first 3 doses 71% Grade 1 29% Grade 2 No Grade ≥ 3 CRS Adverse events attenuate over time
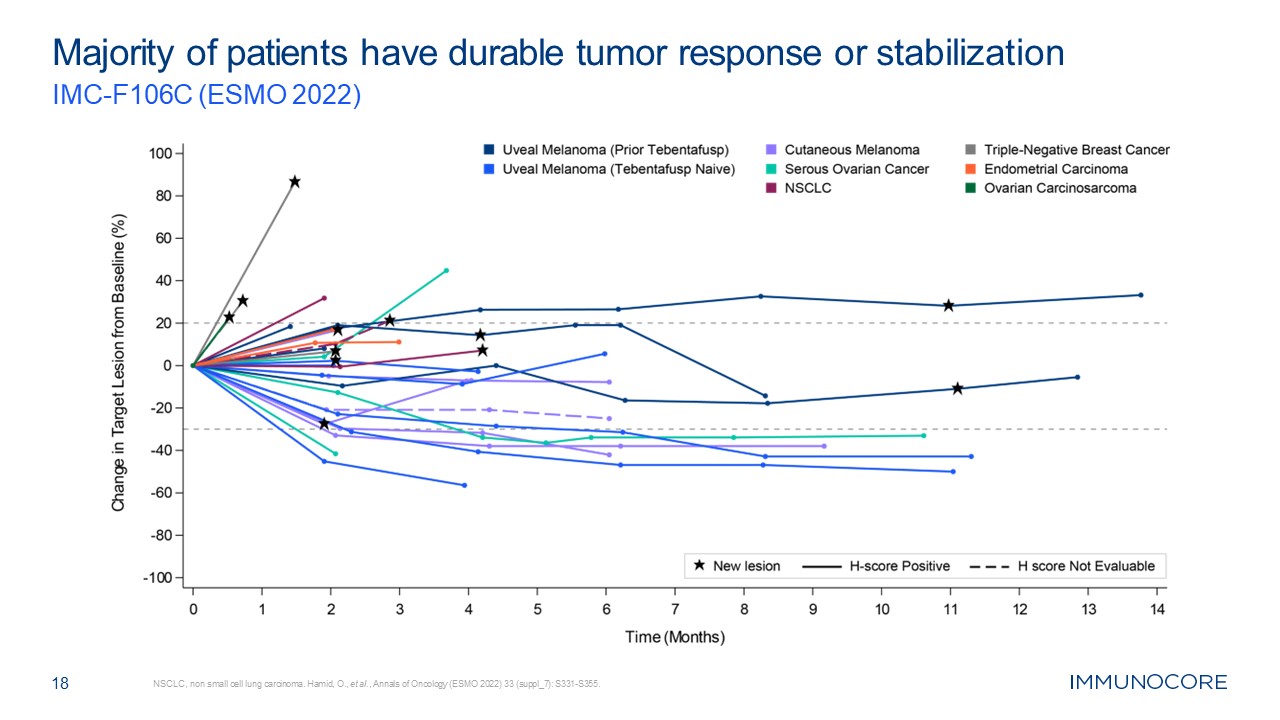
18 Majority of patients have durable tumor response or stabilization IMC-F106C
(ESMO 2022) NSCLC, non small cell lung carcinoma. Hamid, O., et al., Annals of Oncology (ESMO 2022) 33 (suppl_7): S331-S355.
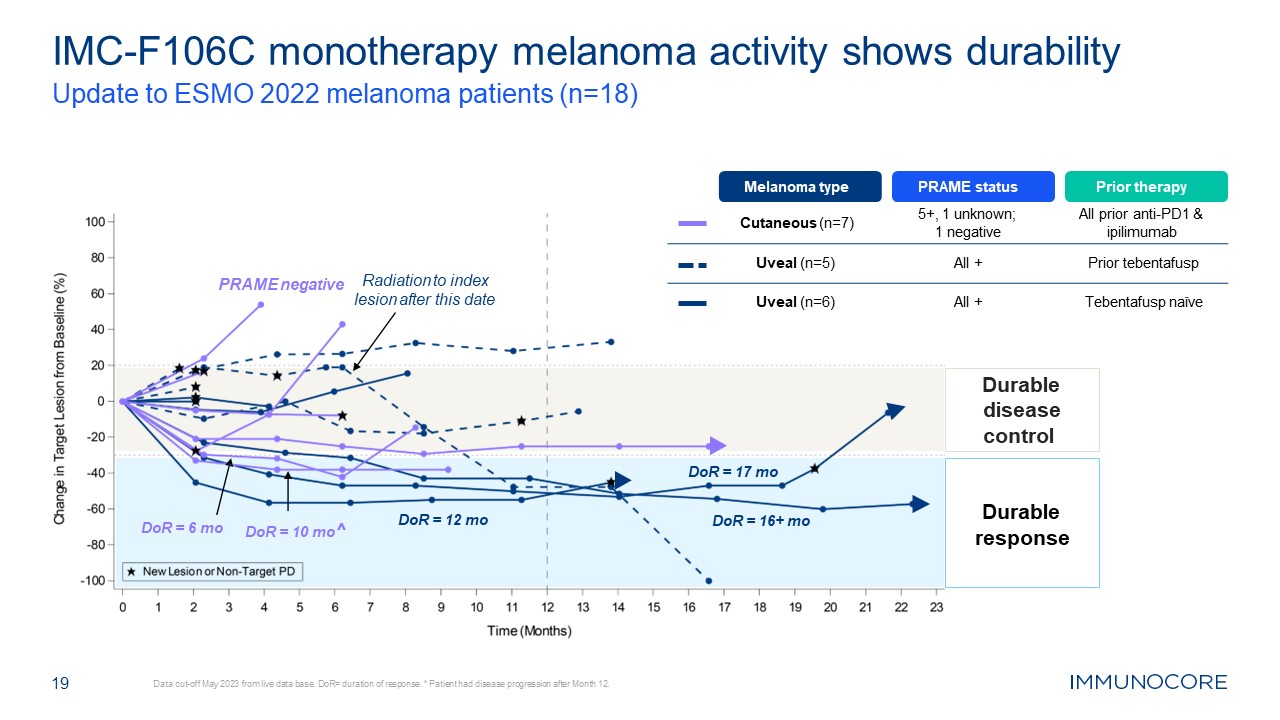
19 IMC-F106C monotherapy melanoma activity shows durability Update to ESMO
2022 melanoma patients (n=18) Data cut-off May 2023 from live data base. DoR= duration of response. ^ Patient had disease progression after Month 12. Durable response Durable disease control Radiation to index lesion after this date DoR
= 10 mo^ DoR = 6 mo DoR = 17 mo DoR = 16+ mo DoR = 12 mo Melanoma type PRAME status Prior therapy Cutaneous (n=7) 5+, 1 unknown; 1 negative All prior anti-PD1 & ipilimumab Uveal (n=5) All + Prior tebentafusp Uveal
(n=6) All + Tebentafusp naïve PRAME negative
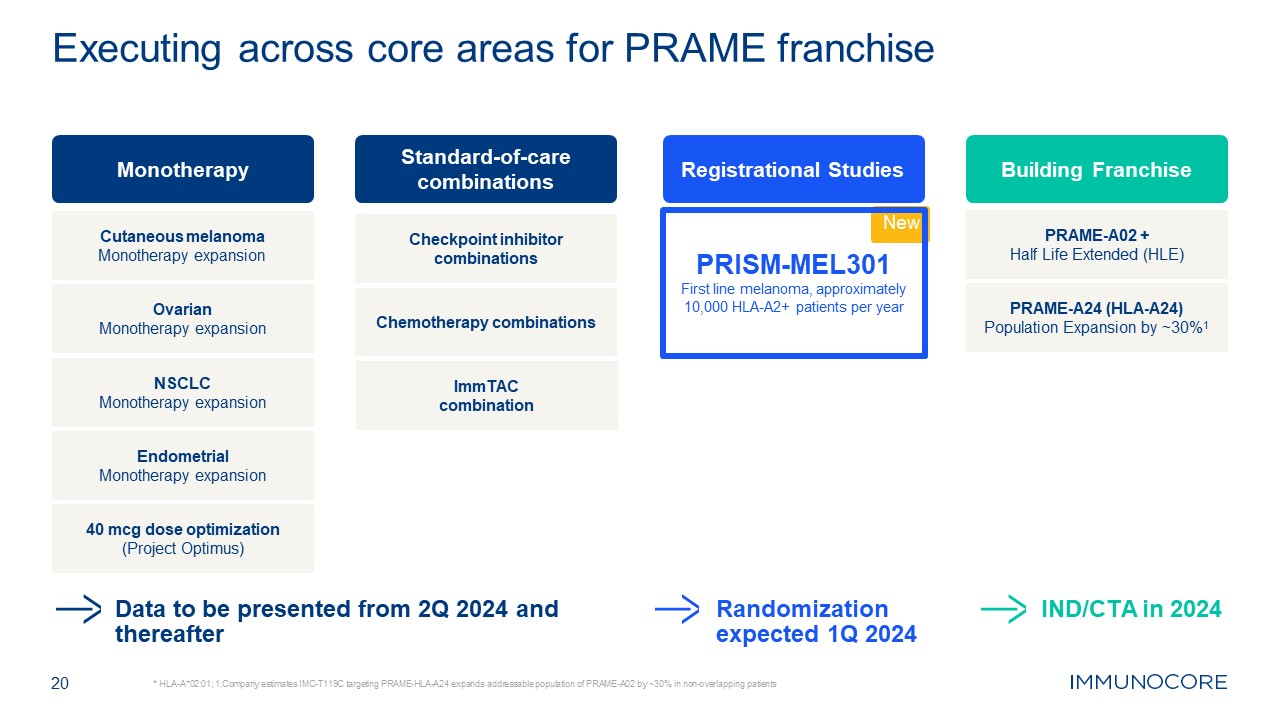
20 * HLA-A*02:01; 1.Company estimates IMC-T119C targeting PRAME-HLA-A24 expands
addressable population of PRAME-A02 by ~30% in non-overlapping patients Executing across core areas for PRAME franchise Data to be presented from 2Q 2024 and thereafter Randomization expected 1Q 2024 Monotherapy Standard-of-care
combinations Checkpoint inhibitor combinations Chemotherapy combinations Endometrial Monotherapy expansion NSCLC Monotherapy expansion Ovarian Monotherapy expansion Cutaneous melanoma Monotherapy expansion 40 mcg dose
optimization (Project Optimus) ImmTAC combination Registrational Studies New PRISM-MEL301 First line melanoma, approximately 10,000 HLA-A2+ patients per year Building Franchise PRAME-A02 + Half Life Extended (HLE) PRAME-A24
(HLA-A24) Population Expansion by ~30%1 IND/CTA in 2024
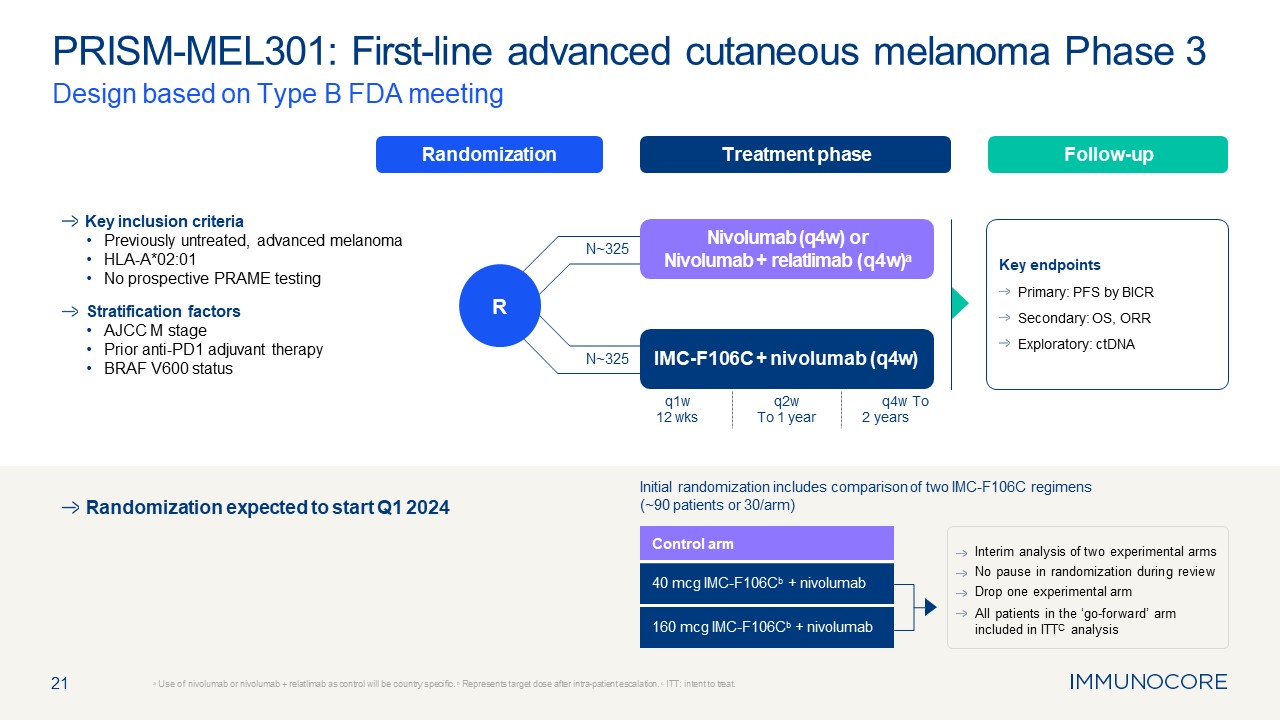
21 PRISM-MEL301: First-line advanced cutaneous melanoma Phase 3 Design based
on Type B FDA meeting a Use of nivolumab or nivolumab + relatlimab as control will be country specific. b Represents target dose after intra-patient escalation. c ITT: intent to treat. Randomization Treatment phase Follow-up R Key
inclusion criteria Previously untreated, advanced melanoma HLA-A*02:01 No prospective PRAME testing Stratification factors AJCC M stage Prior anti-PD1 adjuvant therapy BRAF V600 status Key endpoints Primary: PFS by BICR Secondary:
OS, ORR Exploratory: ctDNA IMC-F106C + nivolumab (q4w) Nivolumab (q4w) or Nivolumab + relatlimab (q4w)a N~325 N~325 q1w 12 wks q2w To 1 year q4w To 2 years Control arm 40 mcg IMC-F106Cb + nivolumab 160 mcg IMC-F106Cb +
nivolumab Interim analysis of two experimental arms No pause in randomization during review Drop one experimental arm All patients in the ‘go-forward’ arm included in ITTC analysis Randomization expected to start Q1 2024 Initial
randomization includes comparison of two IMC-F106C regimens (~90 patients or 30/arm)
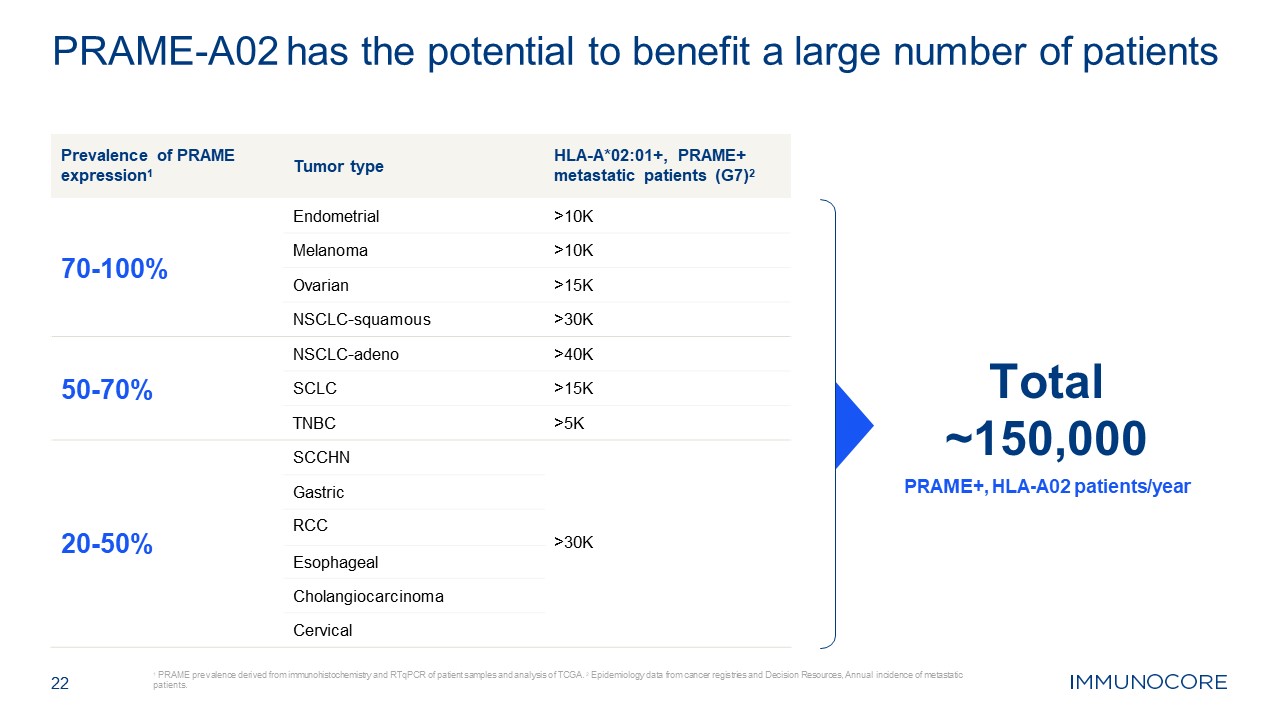
Prevalence of PRAME expression1 Tumor type HLA-A*02:01+, PRAME+ metastatic
patients
(G7)2 70-100% Endometrial >10K Melanoma >10K Ovarian >15K NSCLC-squamous >30K 50-70% NSCLC-adeno >40K SCLC >15K TNBC >5K SCCHN Gastric 20-50% RCC >30K Esophageal Cholangiocarcinoma Cervical 22 1
PRAME prevalence derived from immunohistochemistry and RTqPCR of patient samples and analysis of TCGA. 2 Epidemiology data from cancer registries and Decision Resources, Annual incidence of metastatic patients. PRAME-A02 has the potential to
benefit a large number of patients Total ~150,000 PRAME+, HLA-A02 patients/year

Novel ImmTAC candidate for GI cancers from our discovery engine 23
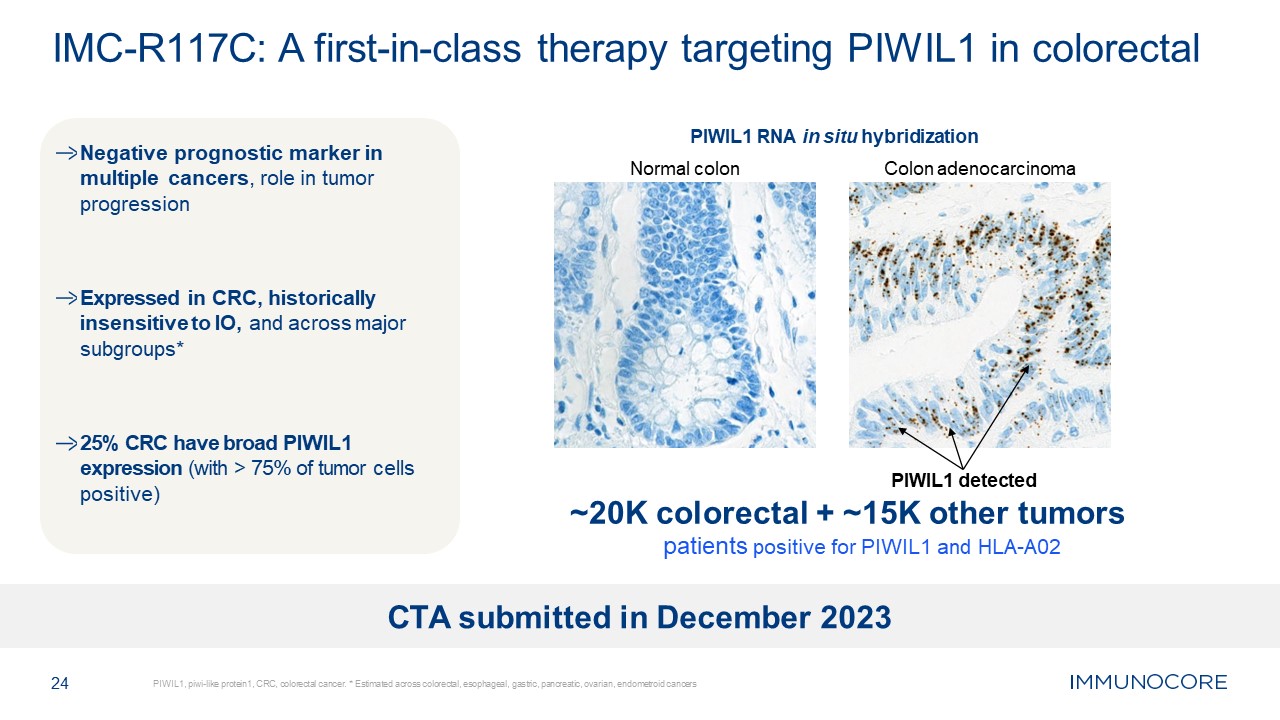
Negative prognostic marker in multiple cancers, role in tumor
progression Expressed in CRC, historically insensitive to IO, and across major subgroups* 25% CRC have broad PIWIL1 expression (with > 75% of tumor cells positive) 24 PIWIL1, piwi-like protein1, CRC, colorectal cancer. * Estimated
across colorectal, esophageal, gastric, pancreatic, ovarian, endometroid cancers IMC-R117C: A first-in-class therapy targeting PIWIL1 in colorectal PIWIL1 RNA in situ hybridization Normal colon Colon adenocarcinoma PIWIL1 detected ~20K
colorectal + ~15K other tumors patients positive for PIWIL1 and HLA-A02 CTA submitted in December 2023

Pursuing a functional cure in infectious diseases
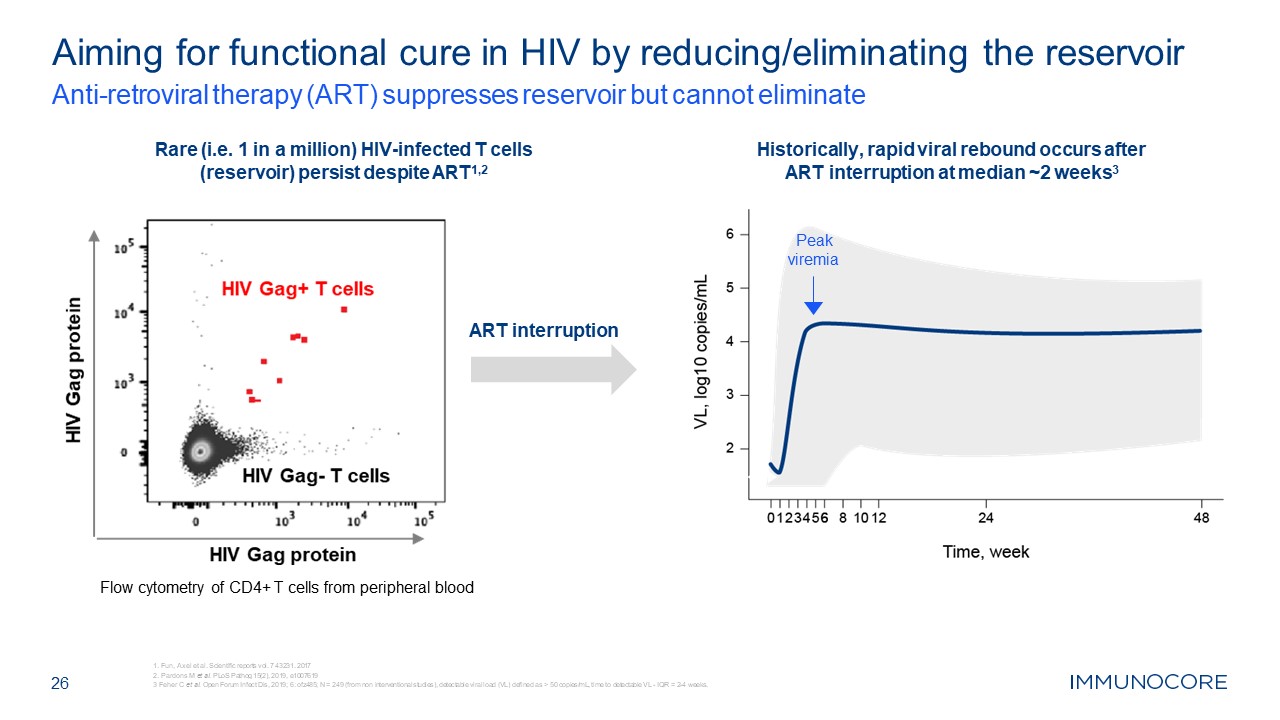
Aiming for functional cure in HIV by reducing/eliminating the
reservoir Anti-retroviral therapy (ART) suppresses reservoir but cannot eliminate 26 Rare (i.e. 1 in a million) HIV-infected T cells (reservoir) persist despite ART1,2 Historically, rapid viral rebound occurs after ART interruption at
median ~2 weeks3 Peak viremia ART interruption Flow cytometry of CD4+ T cells from peripheral blood Fun, Axel et al. Scientific reports vol. 7 43231. 2017 Pardons M et al. PLoS Pathog 15(2), 2019, e1007619 3 Feher C et al. Open Forum
Infect Dis, 2019; 6: ofz485; N = 249 (from non interventional studies), detectable viral load (VL) defined as > 50 copies/mL, time to detectable VL - IQR = 2-4 weeks.
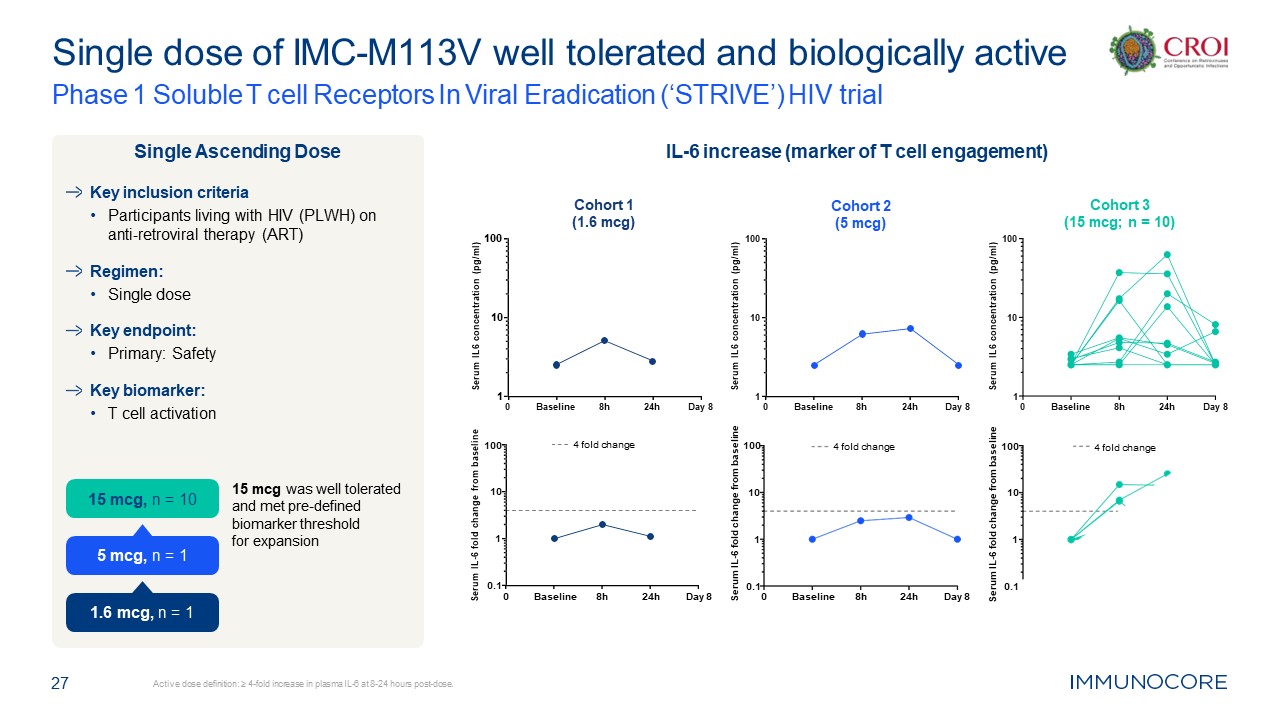
27 Active dose definition: ≥ 4-fold increase in plasma IL-6 at 8-24 hours
post-dose. Single dose of IMC-M113V well tolerated and biologically active Phase 1 Soluble T cell Receptors In Viral Eradication (‘STRIVE’) HIV trial Key inclusion criteria Participants living with HIV (PLWH) on anti-retroviral therapy
(ART) Regimen: Single dose Key endpoint: Primary: Safety Key biomarker: T cell activation 15 mcg was well tolerated and met pre-defined biomarker threshold for expansion Single Ascending Dose IL-6 increase (marker of T cell
engagement) 0 1 10 100 Cohort 2 (5 mcg) Serum IL6 concentration (pg/ml) Baseline 8h 24h Day 8 0 1 10 100 Cohort 1 (1.6 mcg) Serum IL6 concentration (pg/ml) Baseline 8h 24h Day 8 0 1 10 100 Cohort 3 (15 mcg; n =
10) Serum IL6 concentration (pg/ml) Baseline 8h 24h Day 8 0.1 1 10 100 Serum IL-6 fold change from baseline 4 fold change 0 0.1 1 10 100 Serum IL-6 fold change from baseline Baseline 8h 24h Day 8 4 fold
change 0 0.1 1 10 100 Serum IL-6 fold change from baseline Baseline 8h 24h Day 8 4 fold change 15 mcg, n = 10 1.6 mcg, n = 1 5 mcg, n = 1
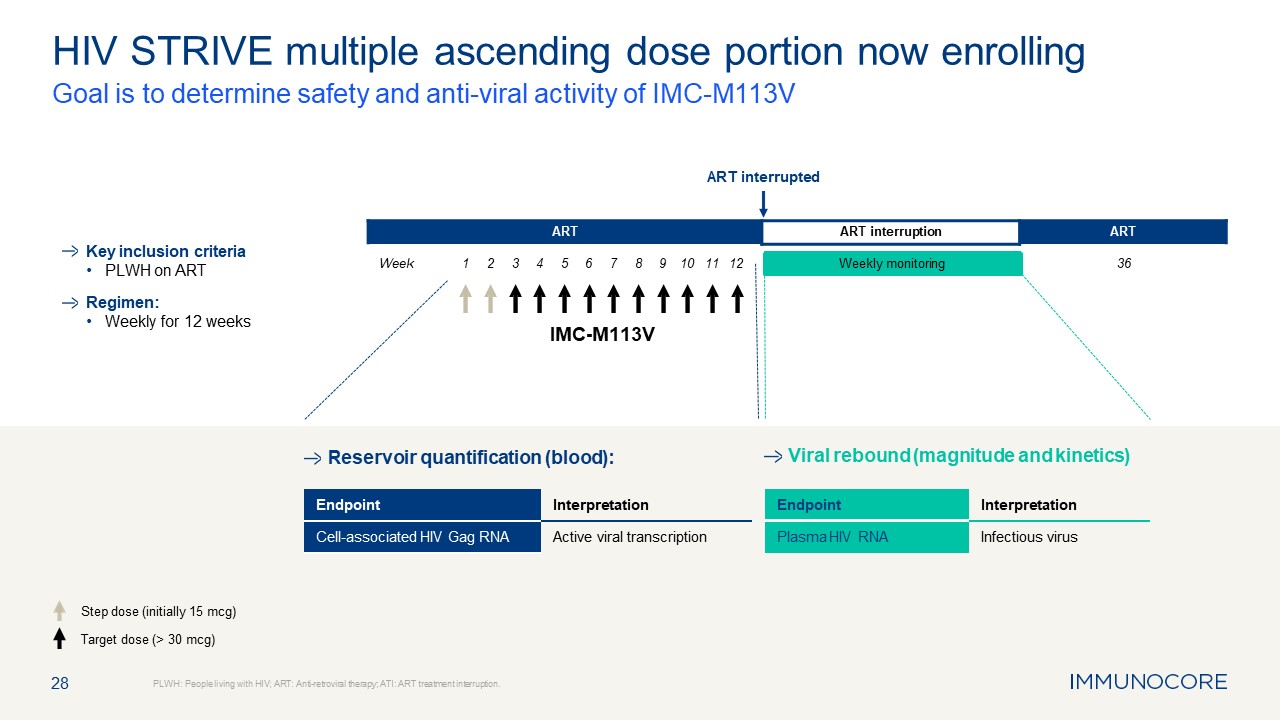
28 HIV STRIVE multiple ascending dose portion now enrolling Goal is to
determine safety and anti-viral activity of IMC-M113V PLWH: People living with HIV; ART: Anti-retroviral therapy; ATI: ART treatment interruption. Key inclusion criteria PLWH on ART Regimen: Weekly for 12 weeks Step dose (initially 15
mcg) Target dose (> 30 mcg) Viral rebound (magnitude and kinetics) IMC-M113V Week ART ART interruption ART ART interrupted Weekly monitoring Reservoir quantification (blood): 1 2 3 4 5 6 7 8 9 10 11
12 36 Endpoint Interpretation Cell-associated HIV Gag RNA Active viral transcription Endpoint Interpretation Plasma HIV RNA Infectious virus
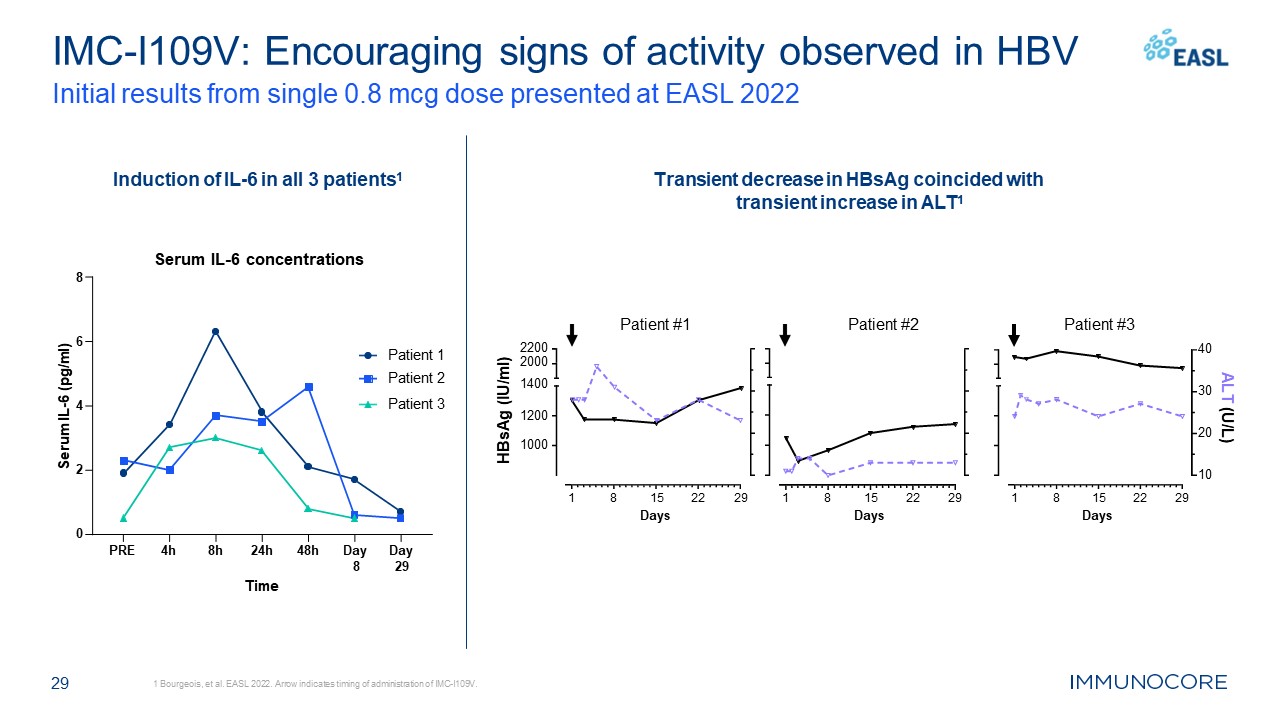
29 IMC-I109V: Encouraging signs of activity observed in HBV Initial results
from single 0.8 mcg dose presented at EASL 2022 1 Bourgeois, et al. EASL 2022. Arrow indicates timing of administration of IMC-I109V. Patient #1 Patient #2 Patient #3 Induction of IL-6 in all 3 patients1 Transient decrease in HBsAg
coincided with transient increase in ALT1 PRE 4h 8h 24h 48h Day Day 8 29 0 2 4 6 Serum IL-6 concentrations 8 Time Serum IL-6 (pg/ml) Patient 1 Patient 2 Patient
3 1 8 22 29 15 Days 1 8 22 29 15 Days 1000 1200 2200 2000 1400 HBsAg (IU/ml) 1 8 22 29 15 Days 10 20 30 40 ALT (U/L)

Pioneering tissue- specific immune suppression for treatment of autoimmune
diseases 30
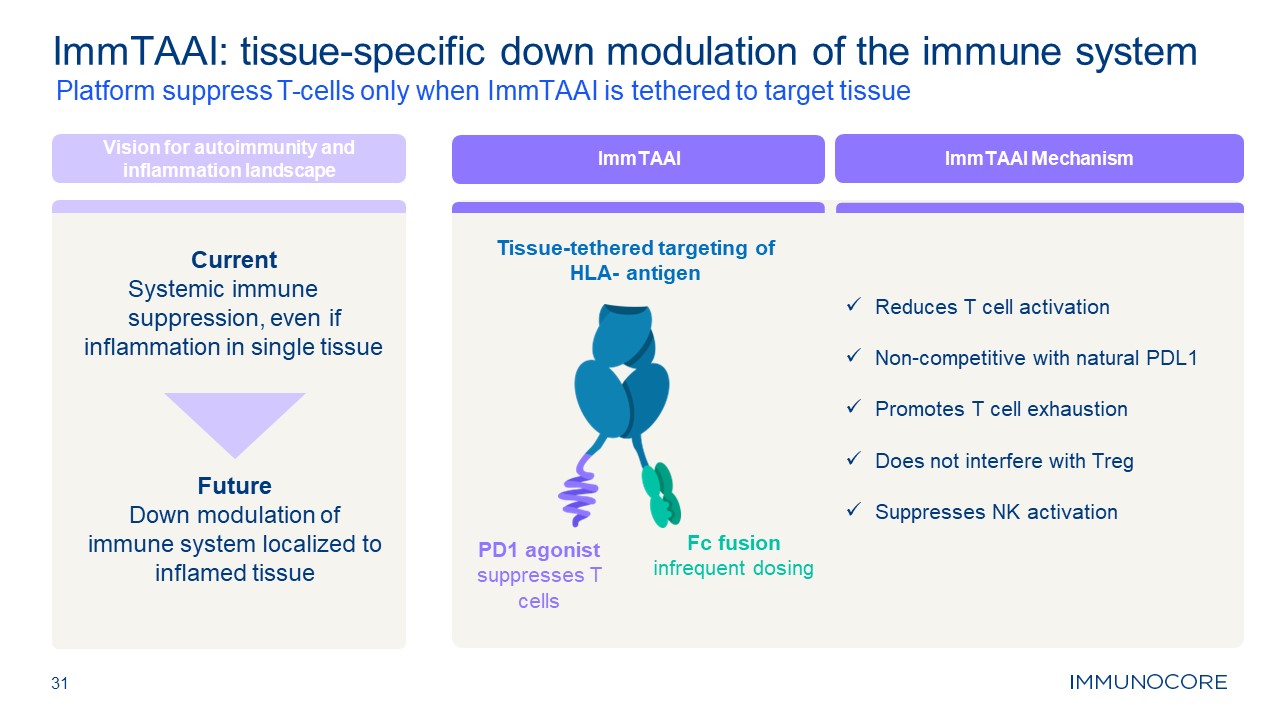
31 Platform suppress T-cells only when ImmTAAI is tethered to target
tissue ImmTAAI: tissue-specific down modulation of the immune system Vision for autoimmunity and inflammation landscape Current Systemic immune suppression, even if inflammation in single tissue Future Down modulation of immune system
localized to inflamed tissue Tissue-tethered targeting of HLA- antigen PD1 agonist suppresses T cells Fc fusion infrequent dosing ImmTAAI ImmTAAI Mechanism Reduces T cell activation Non-competitive with natural PDL1 Promotes T cell
exhaustion Does not interfere with Treg Suppresses NK activation
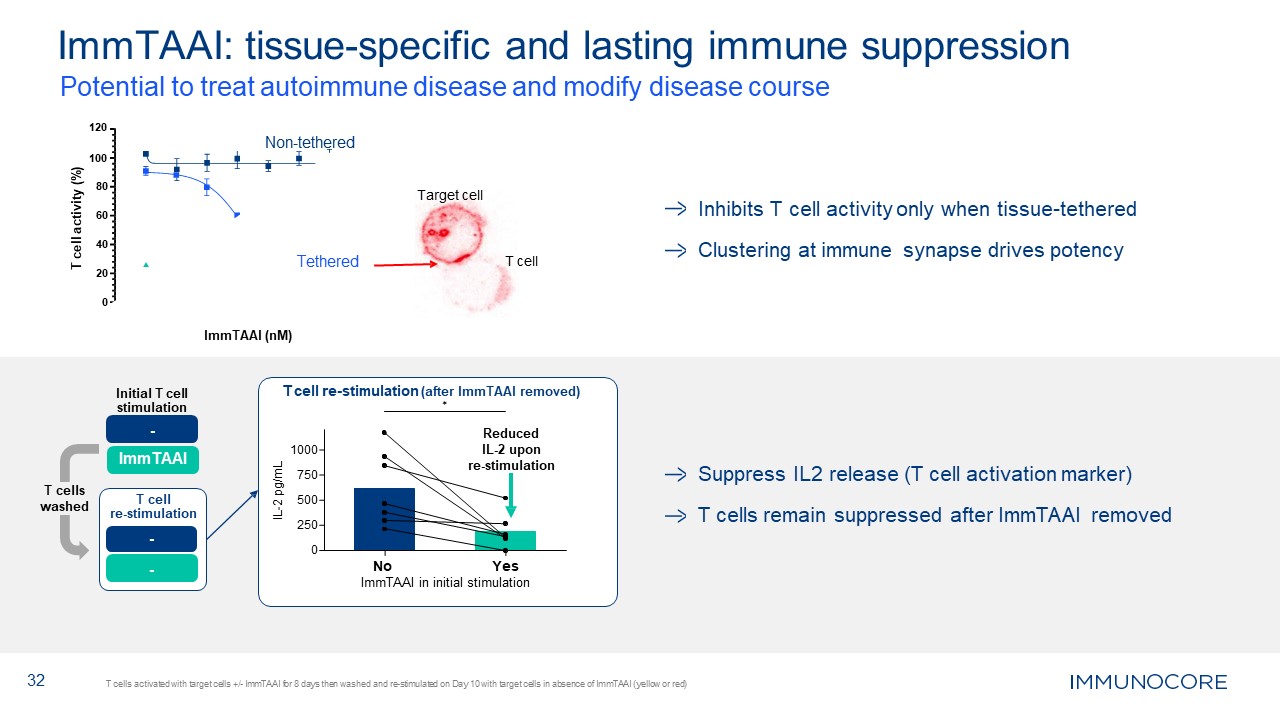
Inhibits T cell activity only when tissue-tethered Clustering at immune synapse
drives potency Suppress IL2 release (T cell activation marker) T cells remain suppressed after ImmTAAI removed 32 ImmTAAI: tissue-specific and lasting immune suppression T cells activated with target cells +/- ImmTAAI for 8 days then
washed and re-stimulated on Day 10 with target cells in absence of ImmTAAI (yellow or red) 0 20 40 60 80 100 Potential to treat autoimmune disease and modify disease course 120 Non-tethered Tethered ImmTAAI (nM) T cell activity
(%) T cell Target cell 250 0 500 1000 750 IL-2 pg/mL T cell re-stimulation (after ImmTAAI removed) ✱ Reduced IL-2 upon re-stimulation No Yes ImmTAAI in initial stimulation T cells washed Initial T cell
stimulation - ImmTAAI - - T cell re-stimulation
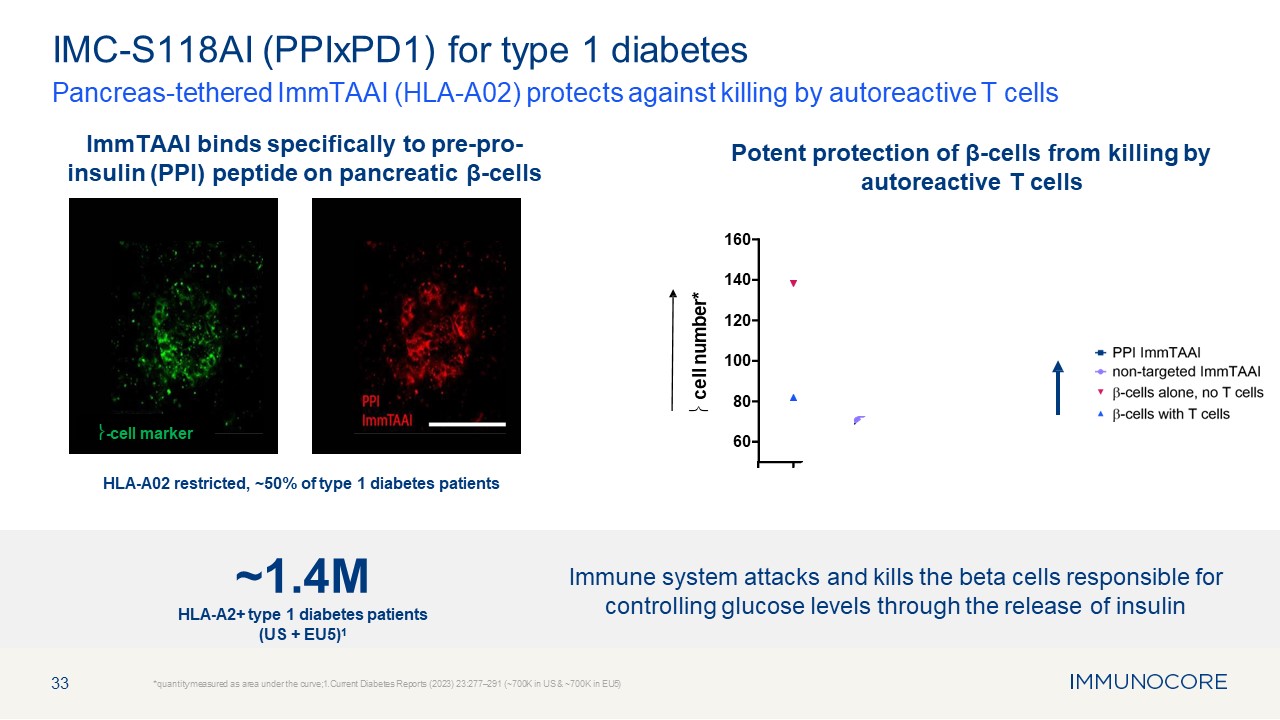
33 Pancreas-tethered ImmTAAI (HLA-A02) protects against killing by autoreactive
T cells *quantity measured as area under the curve;1.Current Diabetes Reports (2023) 23:277–291 (~700K in US & ~700K in EU5) IMC-S118AI (PPIxPD1) for type 1 diabetes HLA-A02 restricted, ~50% of type 1 diabetes
patients 60 80 100 120 140 160 � cell number* ImmTAAI binds specifically to pre-pro- insulin (PPI) peptide on pancreatic β-cells Potent protection of β-cells from killing by autoreactive T cells �-cell marker Immune system attacks
and kills the beta cells responsible for controlling glucose levels through the release of insulin ~1.4M HLA-A2+ type 1 diabetes patients (US + EU5)1
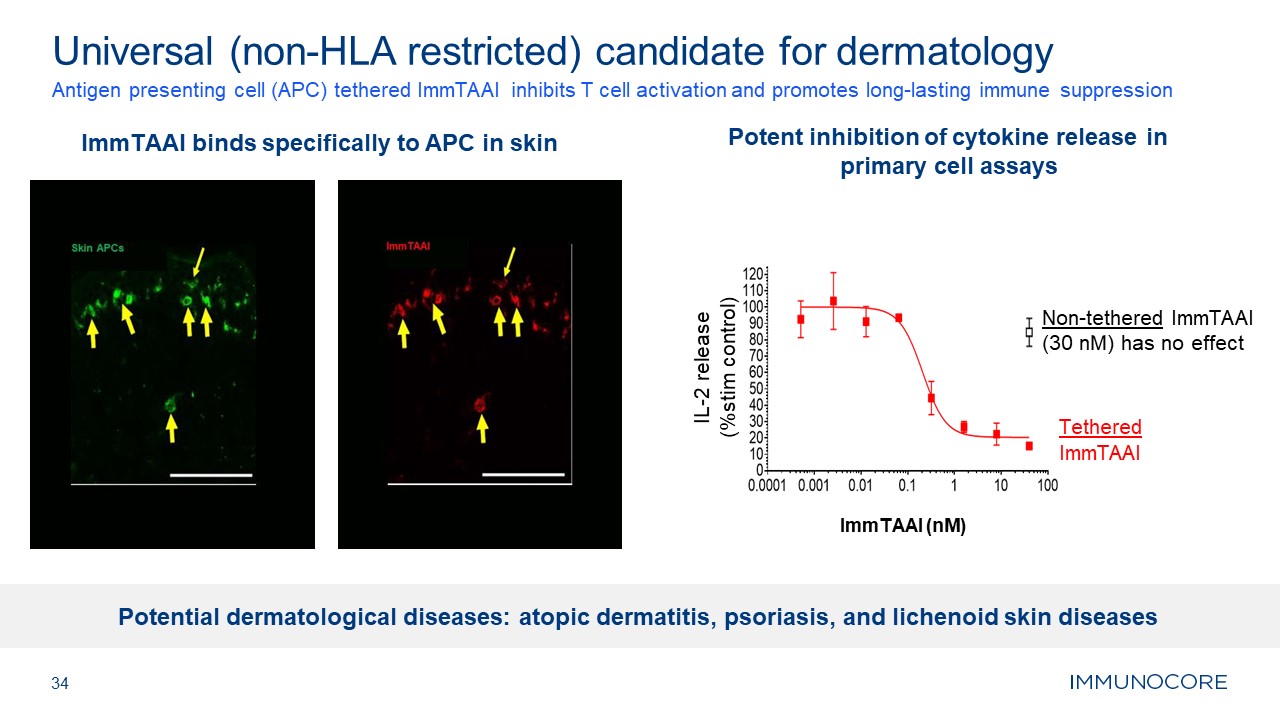
ImmTAAI binds specifically to APC in skin 34 Antigen presenting cell (APC)
tethered ImmTAAI inhibits T cell activation and promotes long-lasting immune suppression Universal (non-HLA restricted) candidate for dermatology Potent inhibition of cytokine release in primary cell assays Non-tethered ImmTAAI (30 nM)
has no effect Tethered ImmTAAI ImmTAAI (nM) IL-2 release (%stim control) Potential dermatological diseases: atopic dermatitis, psoriasis, and lichenoid skin diseases

Upcoming milestones 35

36 Looking ahead Preliminary and unaudited cash position of ~$443 million as
of December 31, 2023 Commercial milestones Clinical milestones KIMMTRAK Continued global growth including commercial launches in Australia and Canada 2024 KIMMTRAK Expansion Topline data from Ph 2 2L+ cutaneous melanoma (TEBE-AM) 4Q
2024 First patient randomized in Ph 3 registrational adjuvant uveal melanoma trial (ATOM); led by EORTC 2H 2024 PRAME Franchise First patient randomized in Ph 3 registrational 1L cutaneous melanoma (PRISM-MEL301) 1Q 2024 Cutaneous
melanoma data from Phase 1 PRAME trial 2Q 2024 Serous ovarian data from Phase 1 PRAME trial 3Q 2024 NSCLC data from Phase 1 PRAME trial 4Q 2024 IND/CTA for PRAME-HLE trial Mid-2024 IND/CTA for PRAME-A24 trial 4Q 2024 PIWIL1 First
patient dosed in PIWIL1 Phase 1 trial 2024 Infectious Diseases Data from Ph 1 HIV MAD/POC trial 2H 2024 Enroll Ph 1 HBV MAD (now including HCC) trial 2024 Autoimmune Diseases Initiating CMC manufacturing for autoimmune candidates 2024

Thank you