Exhibit 99.4

2Q 2023 Financial Results & Business Update Thursday, August 10, 2023

Forward Looking Statements 2 This presentation contains “forward-looking
statements” within the meaning of the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. Words such as “may”, “will”, “believe”, “expect”, “plan”, “anticipate” and similar expressions (as well as other words or
expressions referencing future events or circumstances) are intended to identify forward-looking statements. All statements, other than statements of historical facts, included in this presentation are forward-looking statements. These
statements include, but are not limited to, the commercial performance of KIMMTRAK including planned launches in additional countries; the potential benefits KIMMTRAK will provide for patients; the number of patients Immunocore aims to reach
per year by 2025; the expected submission of investigational new drug applications or clinical trial applications; the potential regulatory approval, expected clinical benefits and availability of Immunocore’s product candidates; expectations
regarding the design, progress, timing, enrollment, scope, expansion, and results of Immunocore’s existing and planned clinical trials; potential growth opportunities and trends, including in connection with product launches in future quarters;
and the Immunocore’s expected cash runway. Any forward-looking statements are based on management’s current expectations and beliefs of future events and are subject to a number of risks and uncertainties that could cause actual events or
results to differ materially and adversely from those set forth in or implied by such forward-looking statements, many of which are beyond the Company’s control. These risks and uncertainties include, but are not limited to, the impact of
worsening macroeconomic conditions on the Company’s business, financial position, strategy and anticipated milestones, including Immunocore’s ability to conduct ongoing and planned clinical trials; Immunocore’s ability to obtain a clinical
supply of current or future product candidates or commercial supply of KIMMTRAK or any future approved products, including as a result of the COVID-19 pandemic, war in Ukraine or global geopolitical tension; Immunocore’s ability to obtain and
maintain regulatory approval of its product candidates, including KIMMTRAK; Immunocore’s ability and plans in continuing to establish and expand a commercial infrastructure and to successfully launch, market and sell KIMMTRAK and any future
approved products; Immunocore’s ability to successfully expand the approved indications for KIMMTRAK or obtain marketing approval for KIMMTRAK in additional geographies in the future; the delay of any current or planned clinical trials, whether
due to patient enrollment delays or otherwise; Immunocore’s ability to successfully demonstrate the safety and efficacy of its product candidates and gain approval of its product candidates on a timely basis, if at all; competition with respect
to market opportunities; unexpected safety or efficacy data observed during preclinical studies or clinical trials; actions of regulatory agencies, which may affect the initiation, timing and progress of clinical trials or future regulatory
approval; Immunocore’s need for and ability to obtain additional funding, on favorable terms or at all, including as a result of worsening macroeconomic conditions, including changes inflation and interest rates and unfavorable general market
conditions, and the impacts thereon of the COVID-19 pandemic, war in Ukraine and global geopolitical tension; Immunocore’s ability to obtain, maintain and enforce intellectual property protection for KIMMTRAK or any product candidates it is
developing; and the success of Immunocore’s current and future collaborations, partnerships or licensing arrangements. These and other risks and uncertainties are described in greater detail in the section titled "Risk Factors" in Immunocore’s
filings with the Securities and Exchange Commission, including Immunocore’s most recent Annual Report on Form 20-F for the year ended December 31, 2022 filed with the Securities and Exchange Commission on March 1, 2023, as well as discussions
of potential risks, uncertainties, and other important factors in the Company’s subsequent filings with the Securities and Exchange Commission. All forward looking statements contained in this presentation speak only as of the date on which
they were made and should not be relied upon as representing its views as of any subsequent date. Except to the extent required by law, Immunocore undertakes no obligation to update such statements to reflect events that occur or circumstances
that exist after the date on which they were made. Such risks may be amplified by pandemics or epidemics, war in Ukraine and related geopolitical tension, and their potential impacts on Immunocore’s business and the overall global economy. All
forward looking statements contained in this presentation speak only as of the date on which they were made and should not be relied upon as representing its views as of any subsequent date. Except to the extent required by law, Immunocore
undertakes no obligation to update such statements to reflect events that occur or circumstances that exist after the date on which they were made. Certain information contained in this presentation relates to or is based on studies,
publications, surveys, and other data obtained from third party sources and Immunocore’s own internal estimates and research. While Immunocore believes these third party sources to be reliable as of the date of this presentation, it has not
independently verified, and makes no representation as to the adequacy, fairness, accuracy, or completeness of, any information obtained from third party sources. KIMMTRAK™ is a trademark owned or licensed to Immunocore.

Agenda 3 Overview & 2Q Highlights Bahija Jallal, PhD – Chief Executive
Officer 2Q Financial Results KIMMTRAK® Commercial Execution Pipeline & PRISM Phase 3 Trial Design Looking Ahead Brian Di Donato – Chief Financial Officer & Head of Strategy Ralph Torbay – Head of Commercial David Berman, MD, PhD
– Head of R&D Bahija Jallal, PhD – Chief Executive Officer Q&A Session

4 Our mission To radically improve outcomes for patients with cancer, infectious
diseases, and autoimmune conditions by pioneering and delivering transformative medicines
1. Projection based on the current business plan, includes projected KIMMTRAK net
revenues. Immunocore may have based this estimate on assumptions that are incorrect and may end up using its resources sooner than anticipated, including as a result of increased costs or milestone payments that may become due. 3. Dollar
amounts based on conversion rate of approximately 1.2709. 5 1H 2023 Highlights Strong KIMMTRAK® performance and pipeline expansion Delivering transformative medicine to patients Executing and Expanding ImmTAC platform in
oncology KIMMTRAK® net revenue $111 million in 1H New launches in Italy, Austria, Finland, and Israel New Phase 3 IMC-F106C (PRAME- A02) 1L cutaneous melanoma trial IMC-F106C-101 Phase 1/2 recruiting patients and data expected in
1H24 Randomization ongoing in KIMMTRAK Ph 2/3 2L+ cutaneous melanoma trial 3 INDs on track for submission over next 18 months Advancing infectious diseases candidates HIV Phase 1 MAD recruiting patients HBV Phase 1 (now includes
hepatocellular carcinoma) recruiting patients

2Q 2023 Financials BRIAN DI DONATO CFO & Head of Strategy 6
1. Projection based on the current business plan, includes projected
KIMMTRAK/tebentafusp net revenues. Immunocore may have based this estimate on assumptions that are incorrect and may end up using its resources sooner than anticipated, including as a result of increased costs or milestone payments that may
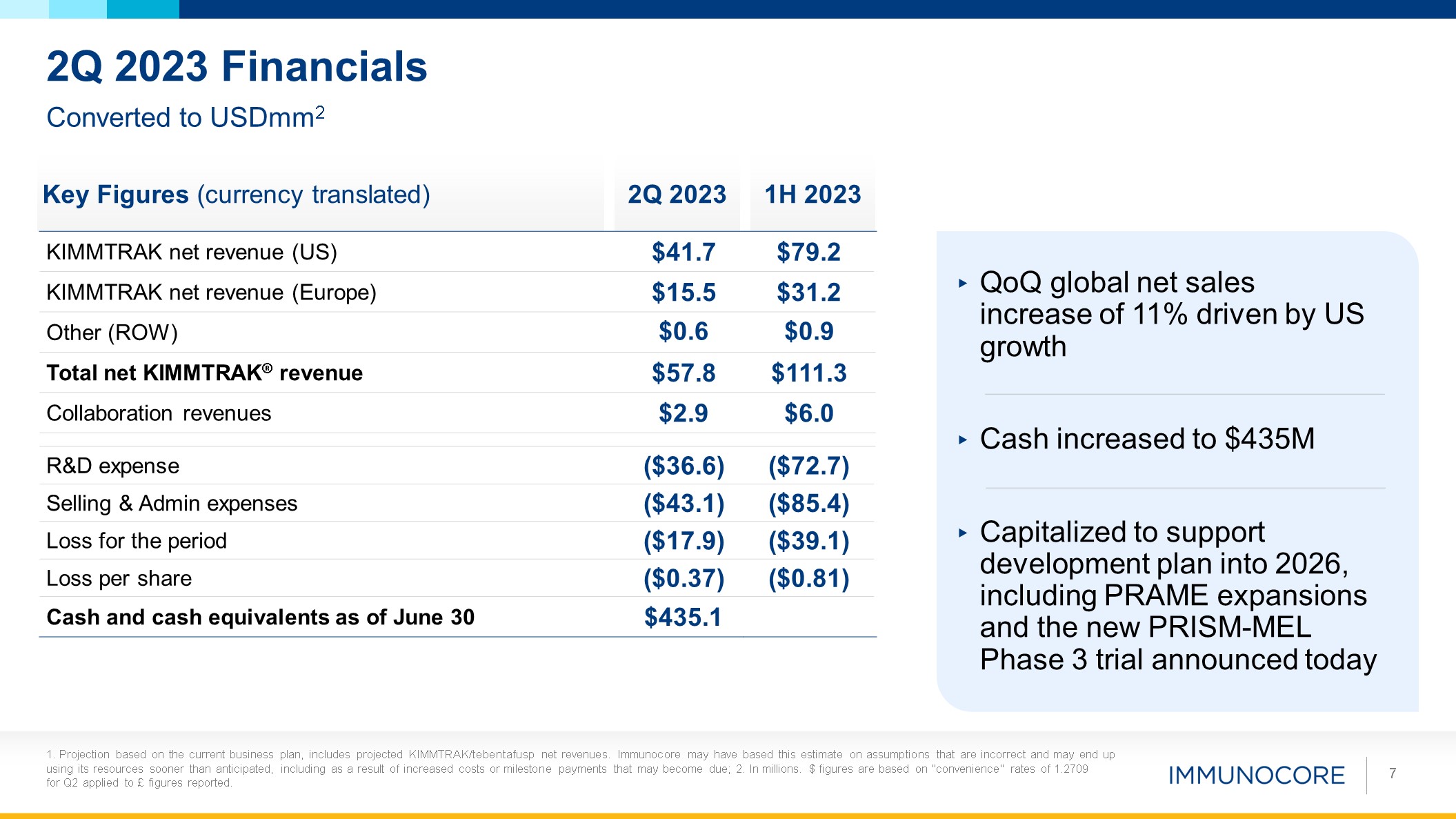
become due; 2. In millions. $ figures are based on "convenience" rates of 1.2709 for Q2 applied to £ figures reported. 7 Converted to USDmm2 2Q 2023 Financials Key Figures (currency translated) 2Q 2023 1H 2023 KIMMTRAK net revenue
(US) $41.7 $79.2 KIMMTRAK net revenue (Europe) $15.5 $31.2 Other (ROW) $0.6 $0.9 Total net KIMMTRAK® revenue $57.8 $111.3 Collaboration revenues $2.9 $6.0 R&D expense ($36.6) ($72.7) Selling & Admin
expenses ($43.1) ($85.4) Loss for the period ($17.9) ($39.1) Loss per share ($0.37) ($0.81) Cash and cash equivalents as of June 30 $435.1 QoQ global net sales increase of 11% driven by US growth Cash increased to $435M Capitalized
to support development plan into 2026, including PRAME expansions and the new PRISM-MEL Phase 3 trial announced today

KIMMTRAK ® Execution RALPH TORBAY Head of Commercial 8
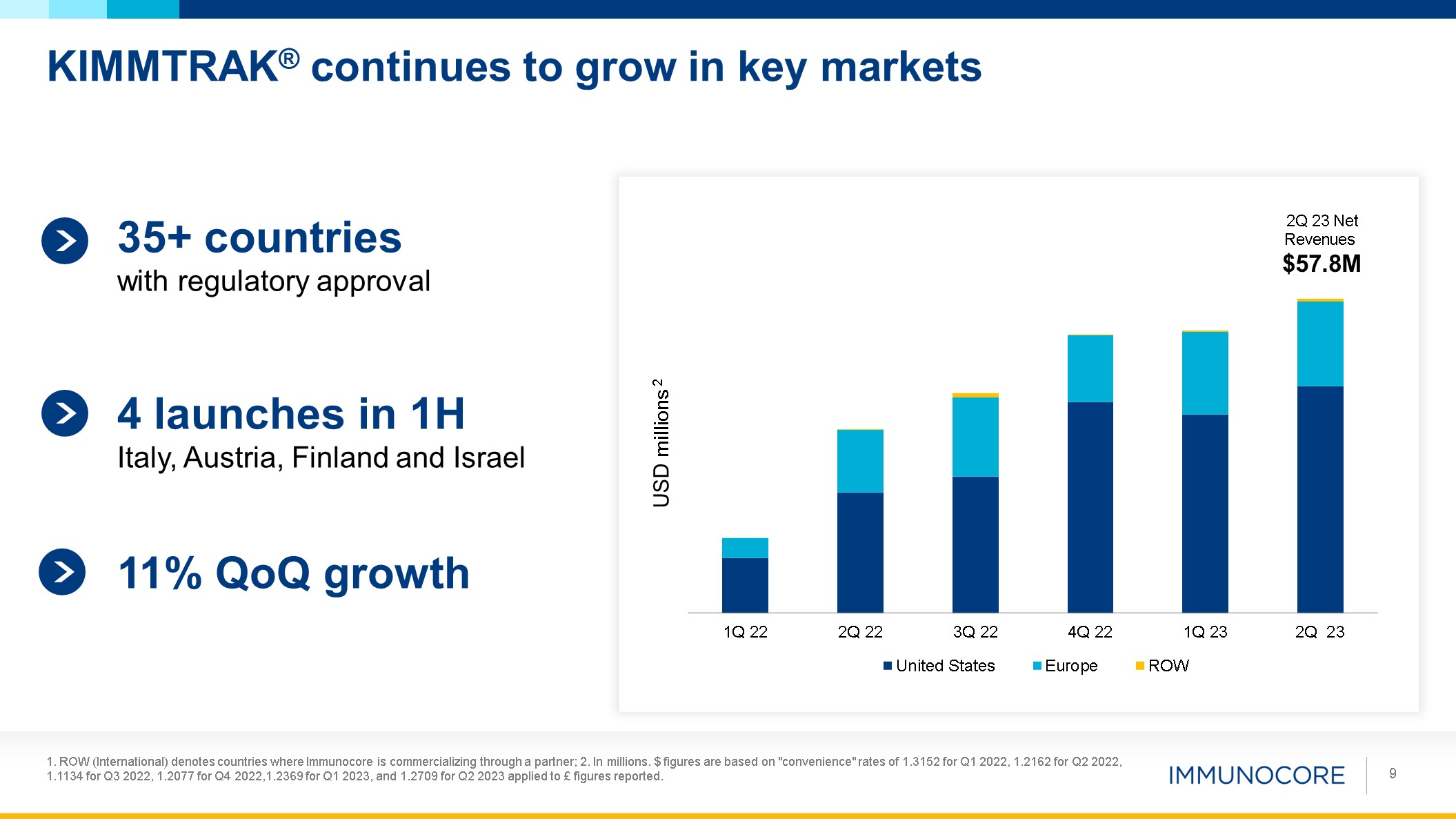
KIMMTRAK® continues to grow in key markets 1. ROW (International) denotes
countries where Immunocore is commercializing through a partner; 2. In millions. $ figures are based on "convenience" rates of 1.3152 for Q1 2022, 1.2162 for Q2 2022, 1.1134 for Q3 2022, 1.2077 for Q4 2022,1.2369 for Q1 2023, and 1.2709 for Q2
2023 applied to £ figures reported. 9 USD millions 2 2Q 23 Net Revenues $57.8M 35+ countries with regulatory approval 4 launches in 1H Italy, Austria, Finland and Israel 11% QoQ growth
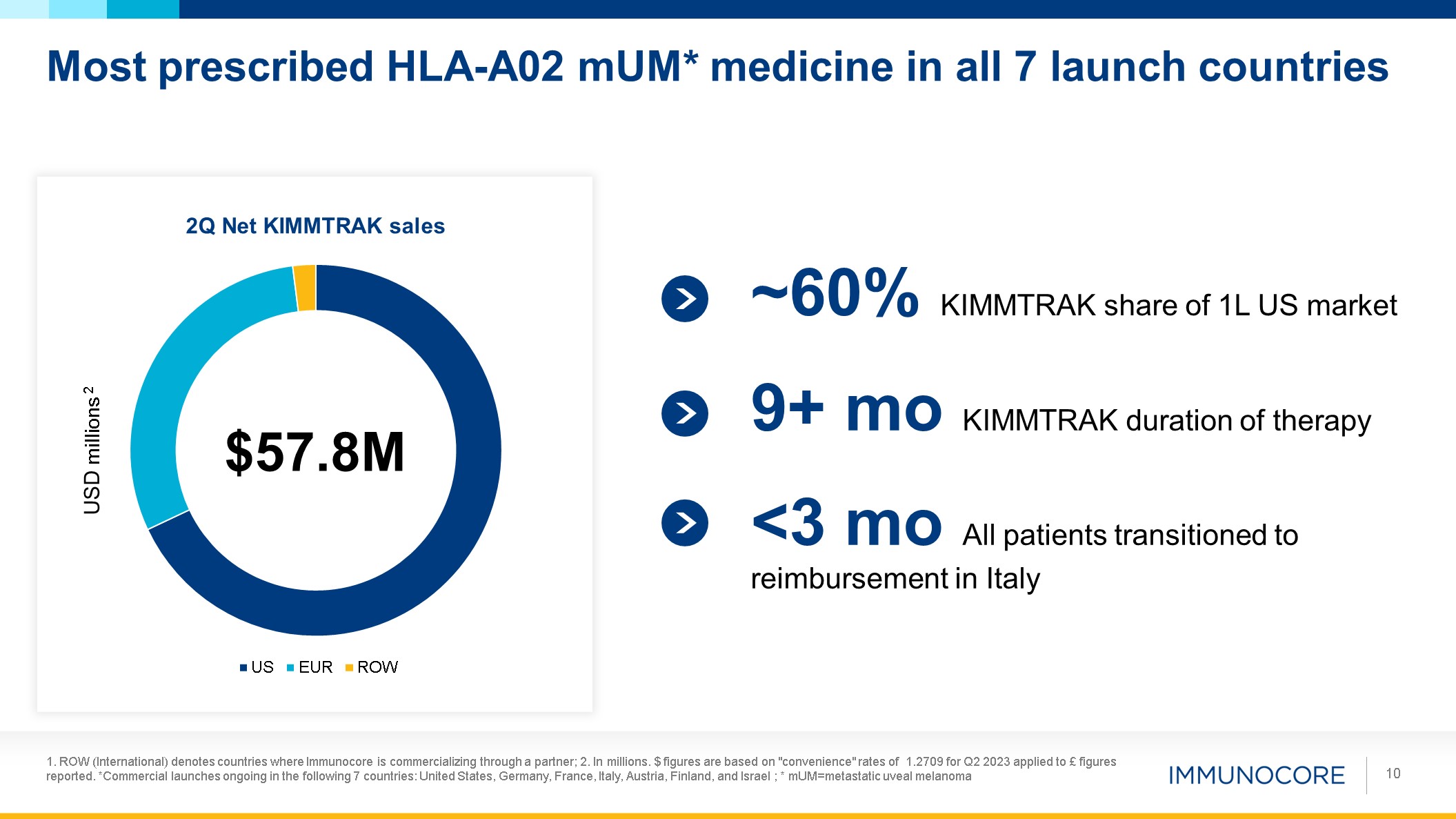
Most prescribed HLA-A02 mUM* medicine in all 7 launch countries 1. ROW
(International) denotes countries where Immunocore is commercializing through a partner; 2. In millions. $ figures are based on "convenience" rates of 1.2709 for Q2 2023 applied to £ figures reported. *Commercial launches ongoing in the
following 7 countries: United States, Germany, France, Italy, Austria, Finland, and Israel ; * mUM=metastatic uveal melanoma 10 USD millions 2 $57.8M ~60% KIMMTRAK share of 1L US market 9+ mo KIMMTRAK duration of therapy <3 mo All
patients transitioned to reimbursement in Italy
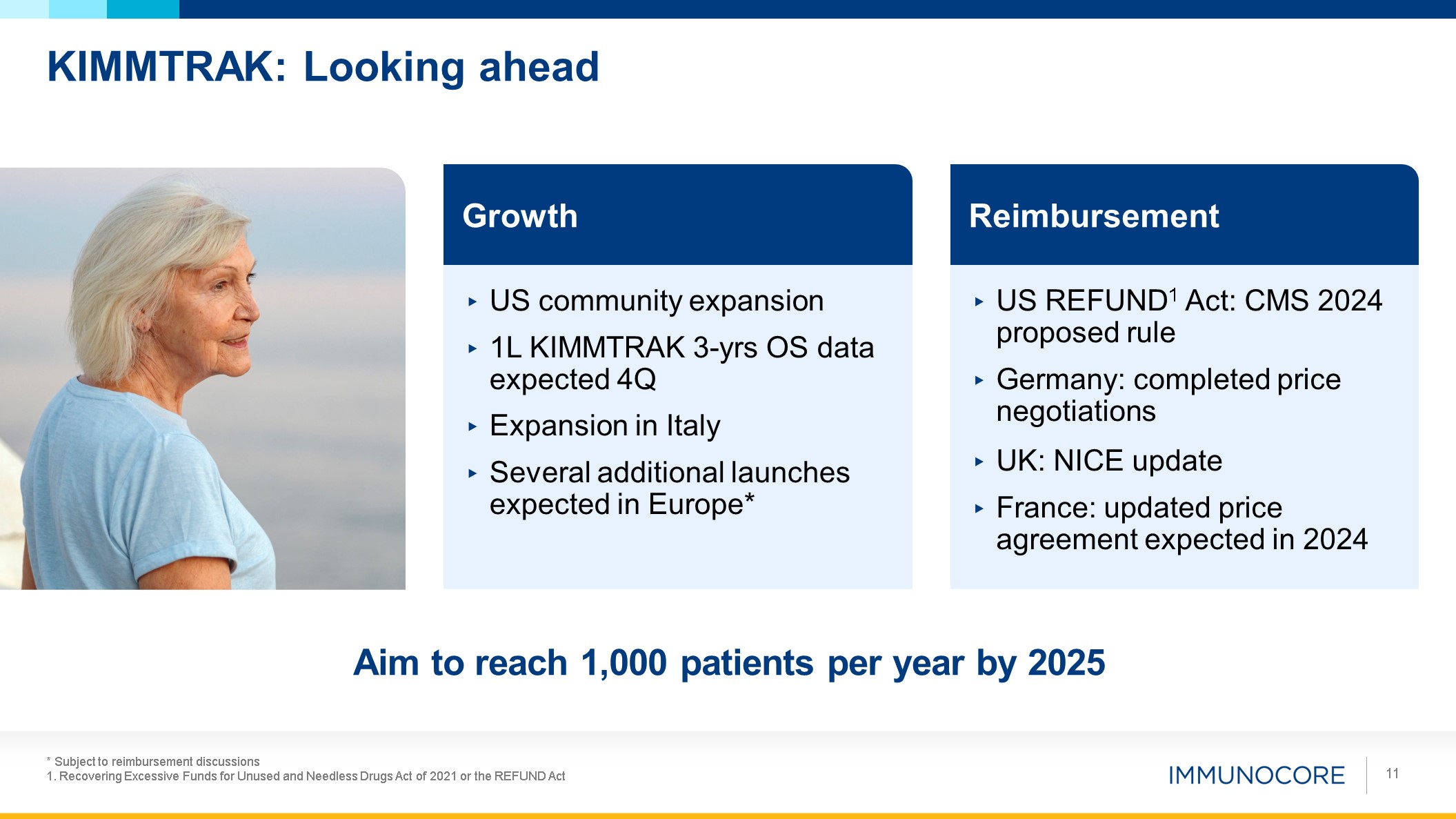
KIMMTRAK: Looking ahead * Subject to reimbursement discussions 1. Recovering
Excessive Funds for Unused and Needless Drugs Act of 2021 or the REFUND Act 11 Growth US community expansion 1L KIMMTRAK 3-yrs OS data expected 4Q Expansion in Italy Several additional launches expected in Europe* Reimbursement US
REFUND1 Act: CMS 2024 proposed rule Germany: completed price negotiations UK: NICE update France: updated price agreement expected in 2024 Aim to reach 1,000 patients per year by 2025

Pipeline & PRISM-MEL301 Trial DAVID BERMAN Head of Research and Development
12
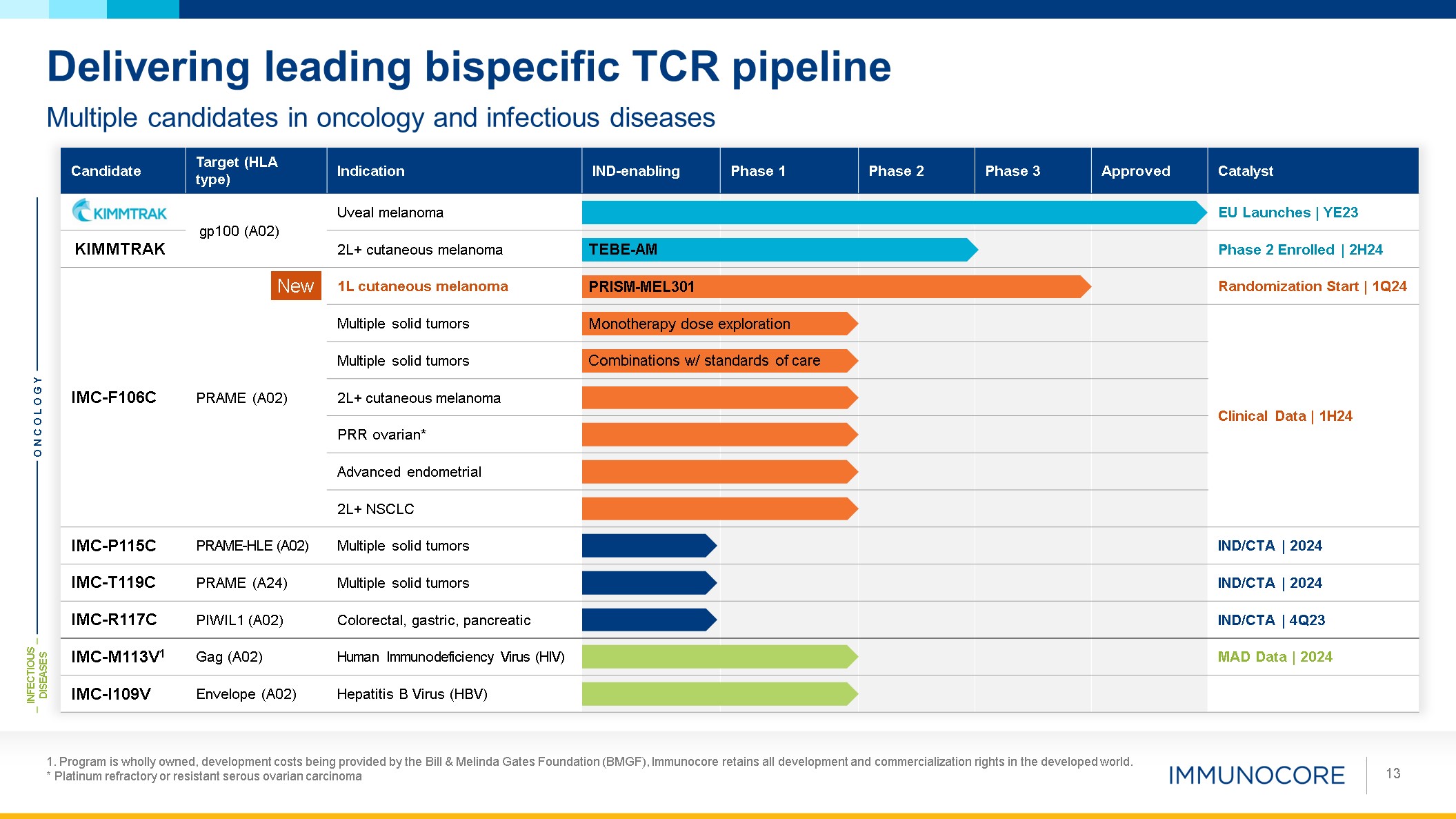
1. Program is wholly owned, development costs being provided by the Bill &
Melinda Gates Foundation (BMGF), Immunocore retains all development and commercialization rights in the developed world. * Platinum refractory or resistant serous ovarian carcinoma 13 Multiple candidates in oncology and infectious
diseases Delivering leading bispecific TCR pipeline Candidate Target (HLA type) Indication IND-enabling Phase 1 Phase 2 Phase 3 Approved Catalyst gp100 (A02) Uveal melanoma EU Launches | YE23 KIMMTRAK 2L+ cutaneous
melanoma Phase 2 Enrolled | 2H24 IMC-F106C PRAME (A02) 1L cutaneous melanoma Randomization Start | 1Q24 Multiple solid tumors Clinical Data | 1H24 Multiple solid tumors 2L+ cutaneous melanoma PRR ovarian* Advanced endometrial 2L+
NSCLC IMC-P115C PRAME-HLE (A02) Multiple solid tumors IND/CTA | 2024 IMC-T119C PRAME (A24) Multiple solid tumors IND/CTA | 2024 IMC-R117C PIWIL1 (A02) Colorectal, gastric, pancreatic IND/CTA | 4Q23 IMC-M113V1 Gag (A02) Human
Immunodeficiency Virus (HIV) MAD Data | 2024 IMC-I109V Envelope (A02) Hepatitis B Virus (HBV) ONCOLOGY INFECTIOUS DISEASES Monotherapy dose exploration Combinations w/ standards of care TEBE-AM PRISM-MEL301 New
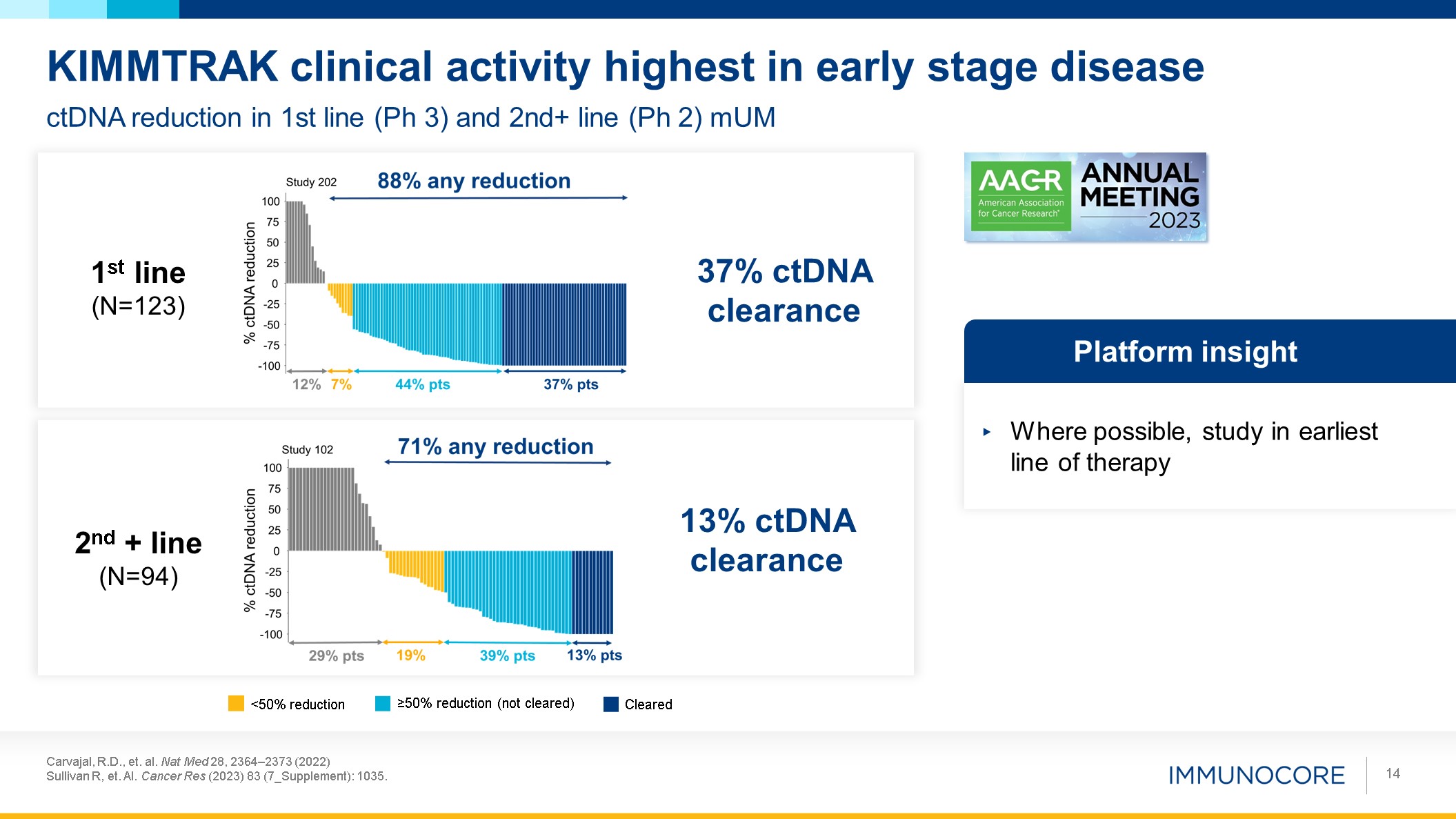
Carvajal, R.D., et. al. Nat Med 28, 2364–2373 (2022) Sullivan R, et. Al. Cancer
Res (2023) 83 (7_Supplement): 1035. 14 ctDNA reduction in 1st line (Ph 3) and 2nd+ line (Ph 2) mUM KIMMTRAK clinical activity highest in early stage disease 1st line (N=123) 2nd + line (N=94) Where possible, study in earliest line of
therapy Platform insight 13% ctDNA clearance 37% ctDNA clearance Cleared ≥50% reduction (not cleared) <50% reduction
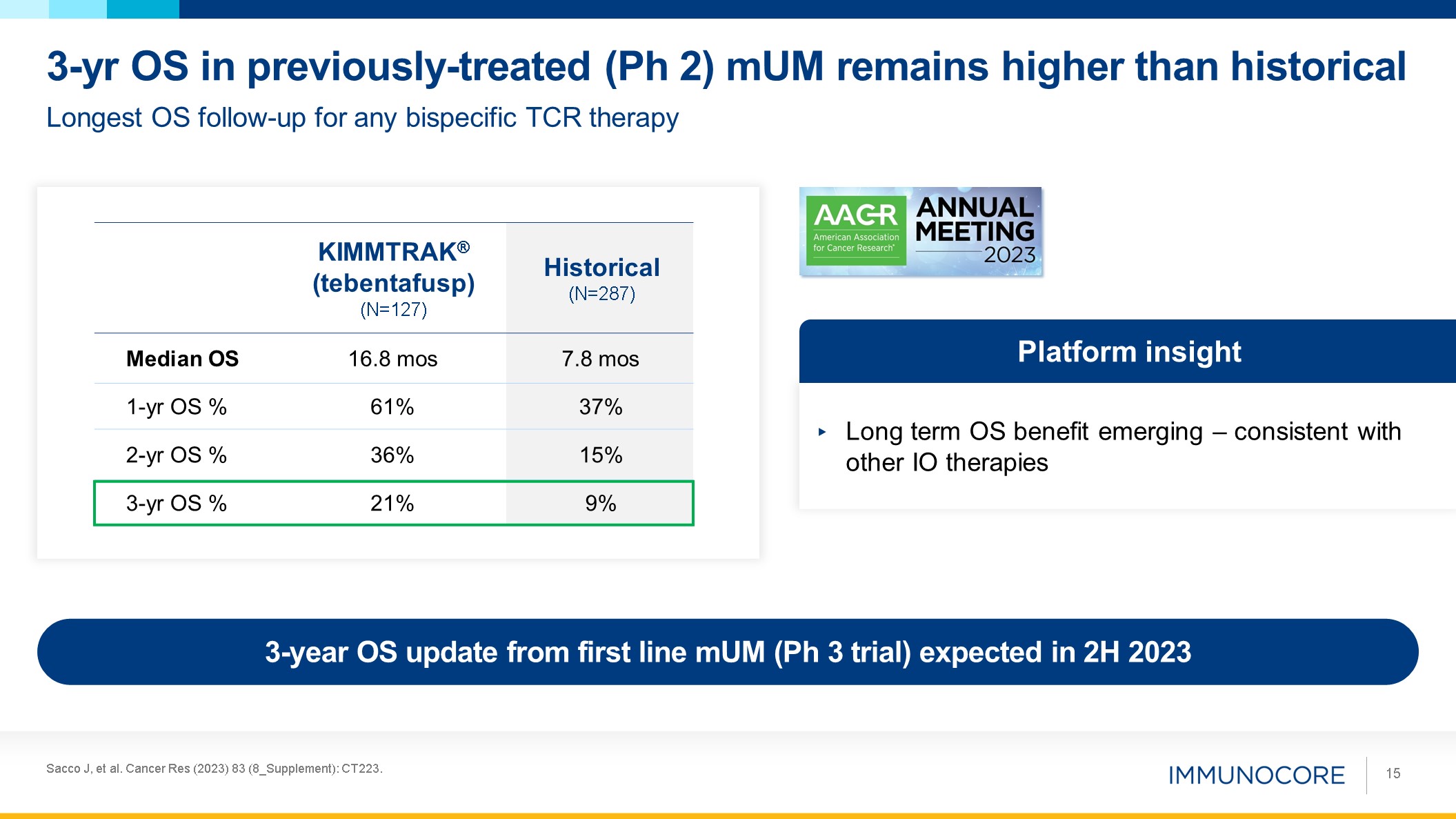
Sacco J, et al. Cancer Res (2023) 83 (8_Supplement): CT223. 15 Longest OS
follow-up for any bispecific TCR therapy 3-yr OS in previously-treated (Ph 2) mUM remains higher than historical Long term OS benefit emerging – consistent with other IO therapies Platform insight KIMMTRAK® (tebentafusp)
(N=127) Historical (N=287) Median OS 16.8 mos 7.8 mos 1-yr OS % 61% 37% 2-yr OS % 36% 15% 3-yr OS % 21% 9% 3-year OS update from first line mUM (Ph 3 trial) expected in 2H 2023
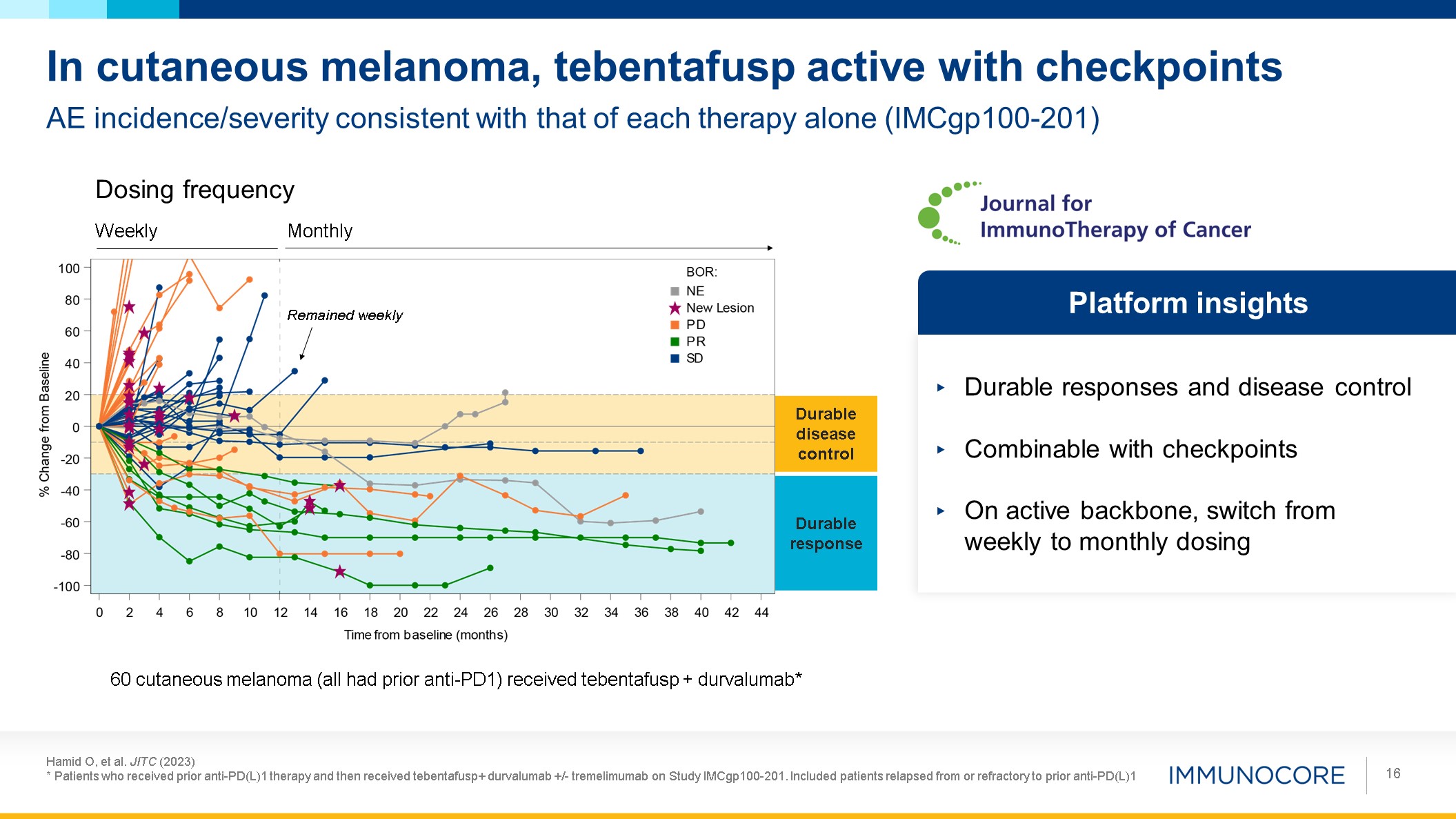
Hamid O, et al. JITC (2023) * Patients who received prior anti-PD(L)1 therapy and
then received tebentafusp+ durvalumab +/- tremelimumab on Study IMCgp100-201. Included patients relapsed from or refractory to prior anti-PD(L)1 16 AE incidence/severity consistent with that of each therapy alone (IMCgp100-201) In cutaneous
melanoma, tebentafusp active with checkpoints Durable responses and disease control Combinable with checkpoints On active backbone, switch from weekly to monthly dosing Platform insights Weekly Monthly Dosing frequency Remained
weekly Durable response Durable disease control 60 cutaneous melanoma (all had prior anti-PD1) received tebentafusp + durvalumab*
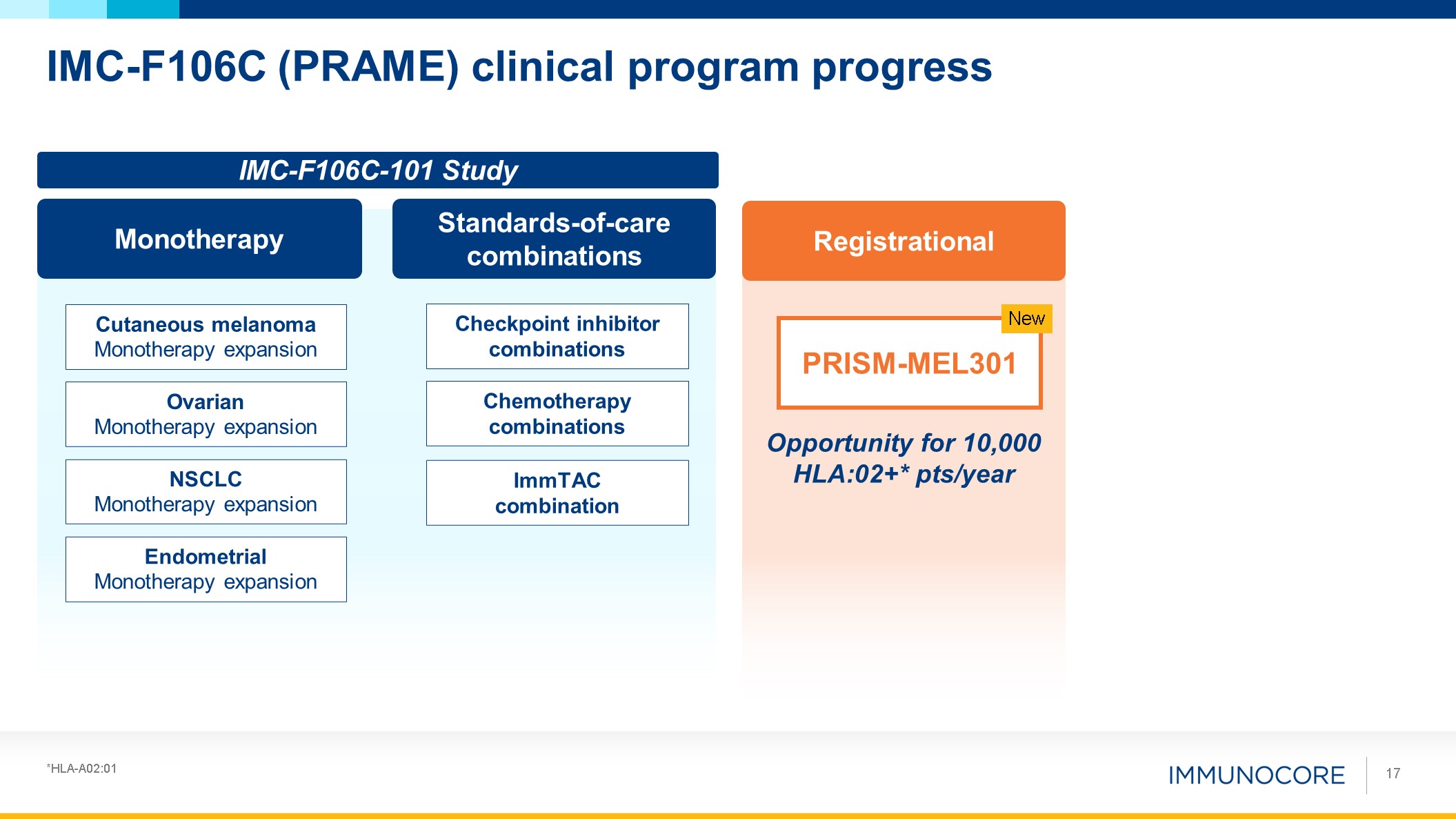
Endometrial Monotherapy expansion NSCLC Monotherapy
expansion Ovarian Monotherapy expansion Cutaneous melanoma Monotherapy expansion IMC-F106C (PRAME) clinical program progress 17 Monotherapy Standards-of-care combinations Checkpoint inhibitor
combinations ImmTAC combination Chemotherapy combinations Registrational PRISM-MEL301 New Opportunity for 10,000 HLA:02+* pts/year IMC-F106C-101 Study *HLA-A02:01
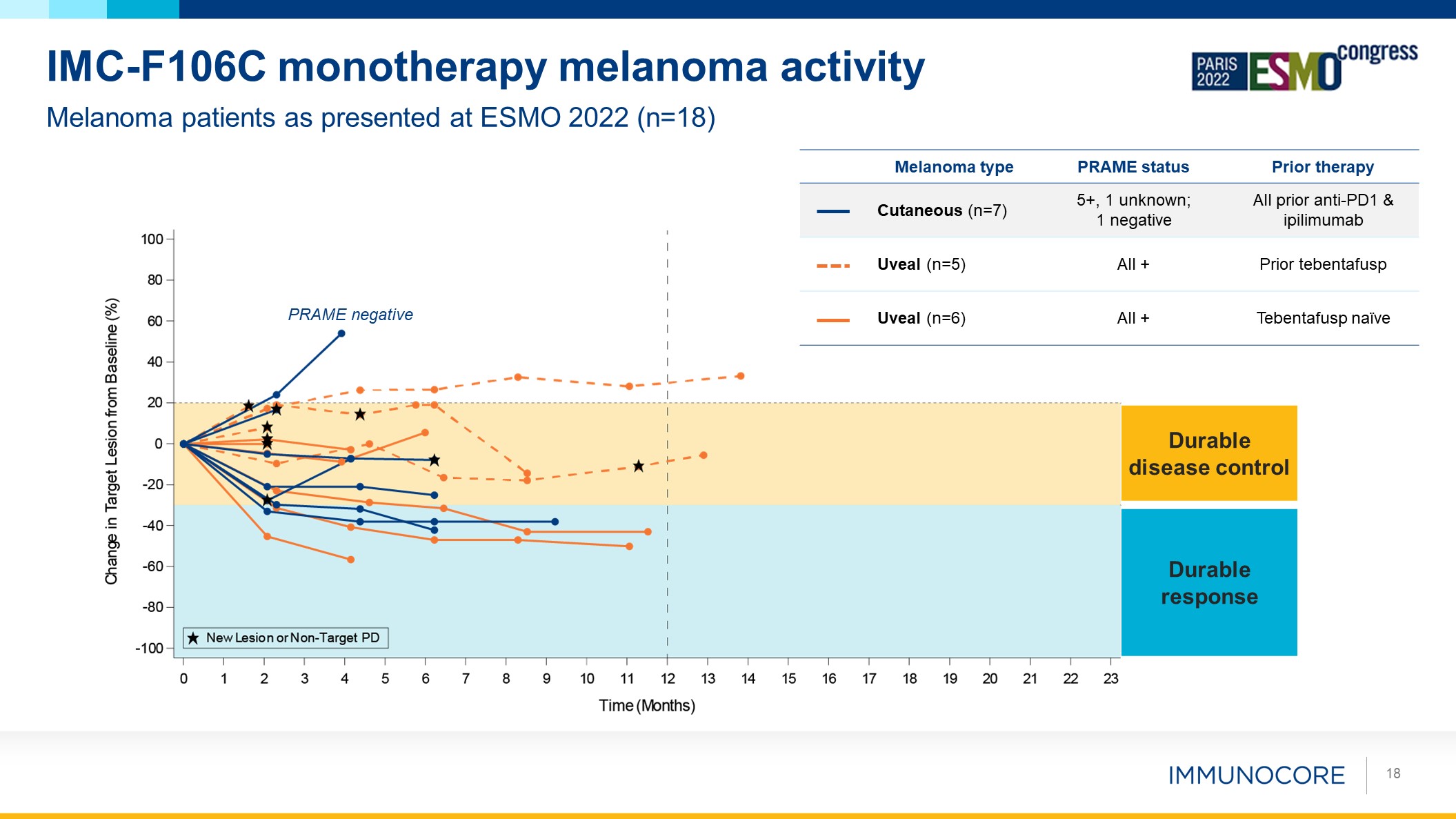
18 Melanoma patients as presented at ESMO 2022 (n=18) IMC-F106C
monotherapy melanoma activity Durable response Durable disease control Melanoma type PRAME status Prior therapy Cutaneous (n=7) 5+, 1 unknown; 1 negative All prior anti-PD1 & ipilimumab Uveal (n=5) All + Prior
tebentafusp Uveal (n=6) All + Tebentafusp naïve PRAME negative
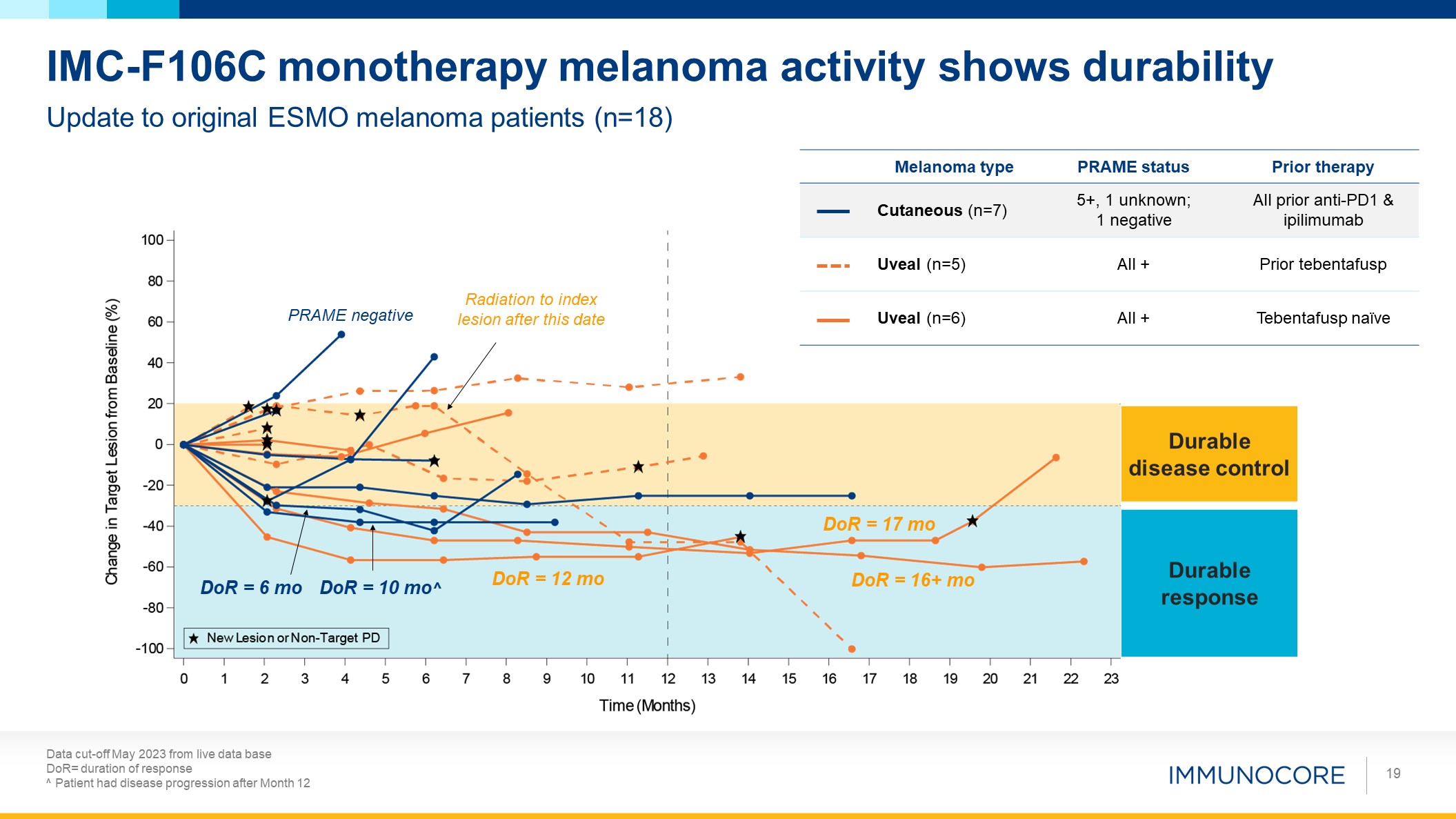
Data cut-off May 2023 from live data base DoR= duration of response ^
Patient had disease progression after Month 12 19 Update to original ESMO melanoma patients (n=18) IMC-F106C monotherapy melanoma activity shows durability Durable response Radiation to index lesion after this date DoR = 10 mo^ DoR
= 6 mo DoR = 17 mo DoR = 16+ mo DoR = 12 mo PRAME negative Durable disease control Melanoma type PRAME status Prior therapy Cutaneous (n=7) 5+, 1 unknown; 1 negative All prior anti-PD1 & ipilimumab Uveal (n=5) All
+ Prior tebentafusp Uveal (n=6) All + Tebentafusp naïve
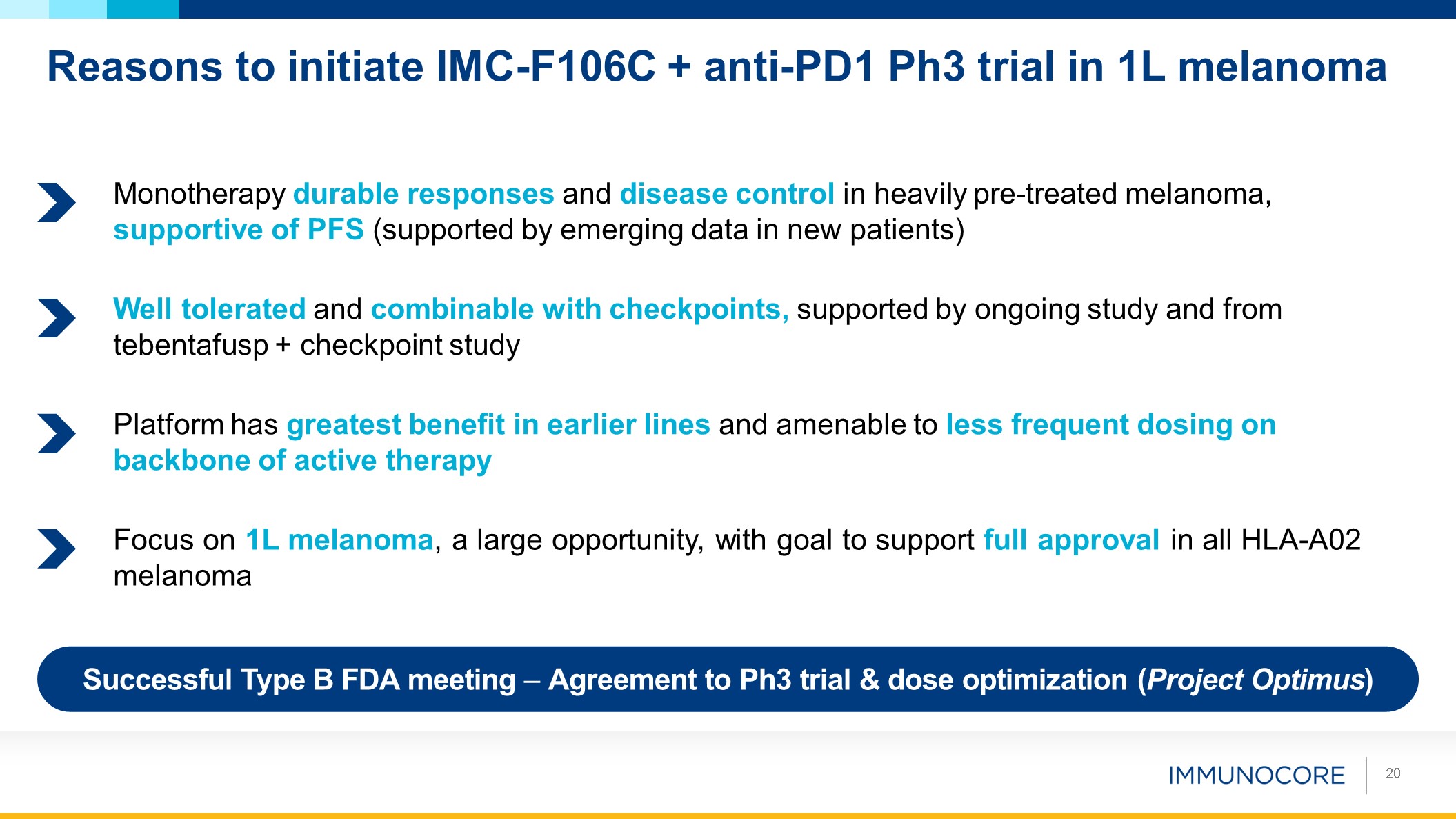
Reasons to initiate IMC-F106C + anti-PD1 Ph3 trial in 1L melanoma 20 Successful
Type B FDA meeting – Agreement to Ph3 trial & dose optimization (Project Optimus) Monotherapy durable responses and disease control in heavily pre-treated melanoma, supportive of PFS (supported by emerging data in new patients) Well
tolerated and combinable with checkpoints, supported by ongoing study and from tebentafusp + checkpoint study Focus on 1L melanoma, a large opportunity, with goal to support full approval in all HLA‑A02 melanoma Platform has greatest
benefit in earlier lines and amenable to less frequent dosing on backbone of active therapy
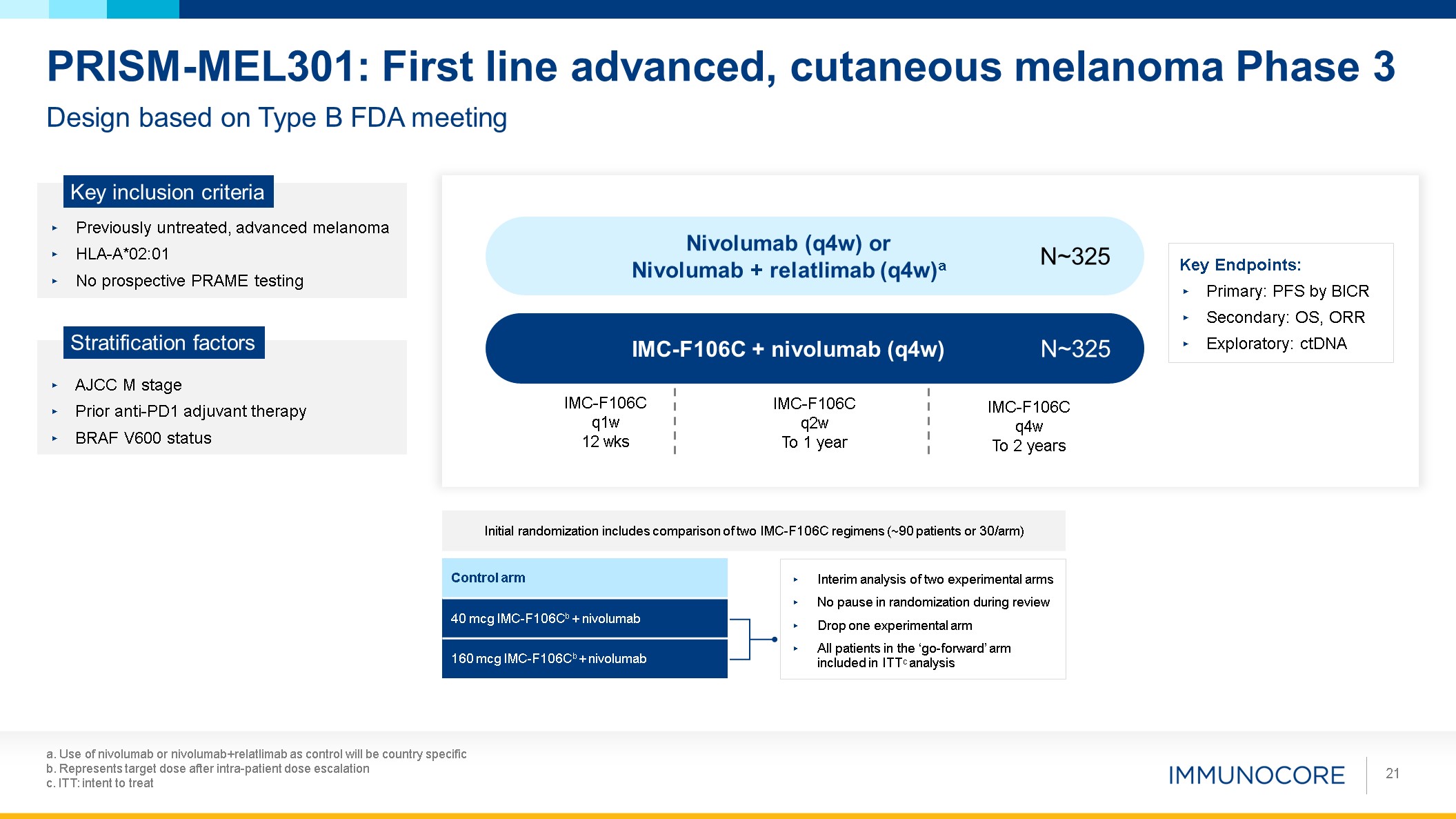
a. Use of nivolumab or nivolumab+relatlimab as control will be country
specific b. Represents target dose after intra-patient dose escalation c. ITT: intent to treat 21 Design based on Type B FDA meeting PRISM-MEL301: First line advanced, cutaneous melanoma Phase 3 Previously untreated, advanced
melanoma HLA-A*02:01 No prospective PRAME testing Key inclusion criteria AJCC M stage Prior anti-PD1 adjuvant therapy BRAF V600 status Stratification factors IMC-F106C + nivolumab (q4w) Nivolumab (q4w) or Nivolumab + relatlimab
(q4w)a N~325 N~325 Key Endpoints: Primary: PFS by BICR Secondary: OS, ORR Exploratory: ctDNA IMC-F106C q1w 12 wks IMC-F106C q2w To 1 year IMC-F106C q4w To 2 years Control arm 40 mcg IMC-F106Cb + nivolumab 160 mcg IMC-F106Cb +
nivolumab Initial randomization includes comparison of two IMC-F106C regimens (~90 patients or 30/arm) Interim analysis of two experimental arms No pause in randomization during review Drop one experimental arm All patients in the
‘go-forward’ arm included in ITTC analysis
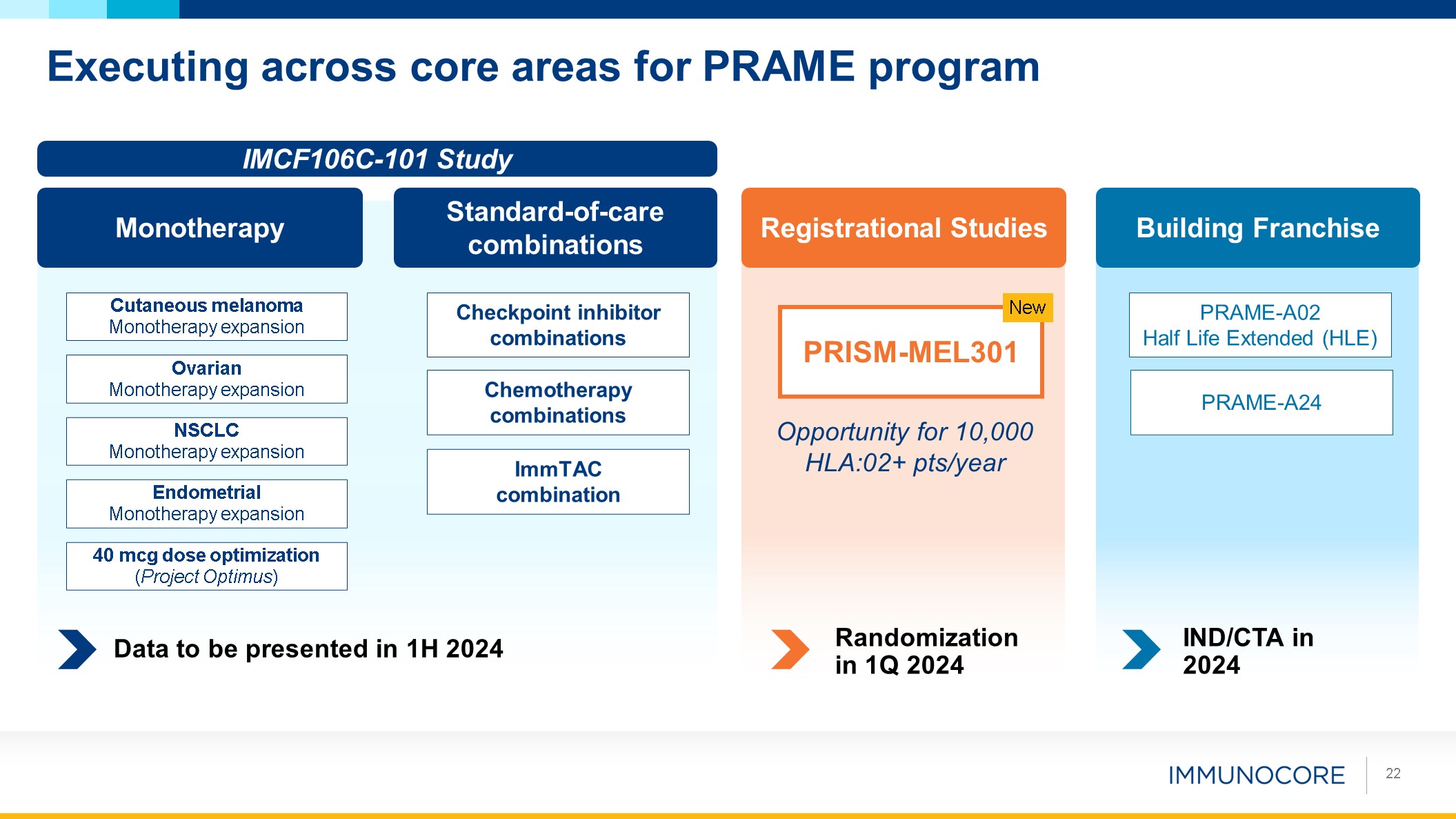
Executing across core areas for PRAME program 22 Endometrial Monotherapy
expansion NSCLC Monotherapy expansion Ovarian Monotherapy expansion Cutaneous melanoma Monotherapy expansion Monotherapy Standard-of-care combinations Checkpoint inhibitor combinations Chemotherapy combinations 40 mcg dose
optimization (Project Optimus) IMCF106C-101 Study ImmTAC combination Data to be presented in 1H 2024 Registrational Studies PRISM-MEL301 New Opportunity for 10,000 HLA:02+ pts/year Randomization in 1Q 2024 Building
Franchise PRAME-A02 Half Life Extended (HLE) PRAME-A24 IND/CTA in 2024

Looking Ahead Bahija Jallal Chief Executive Officer 23
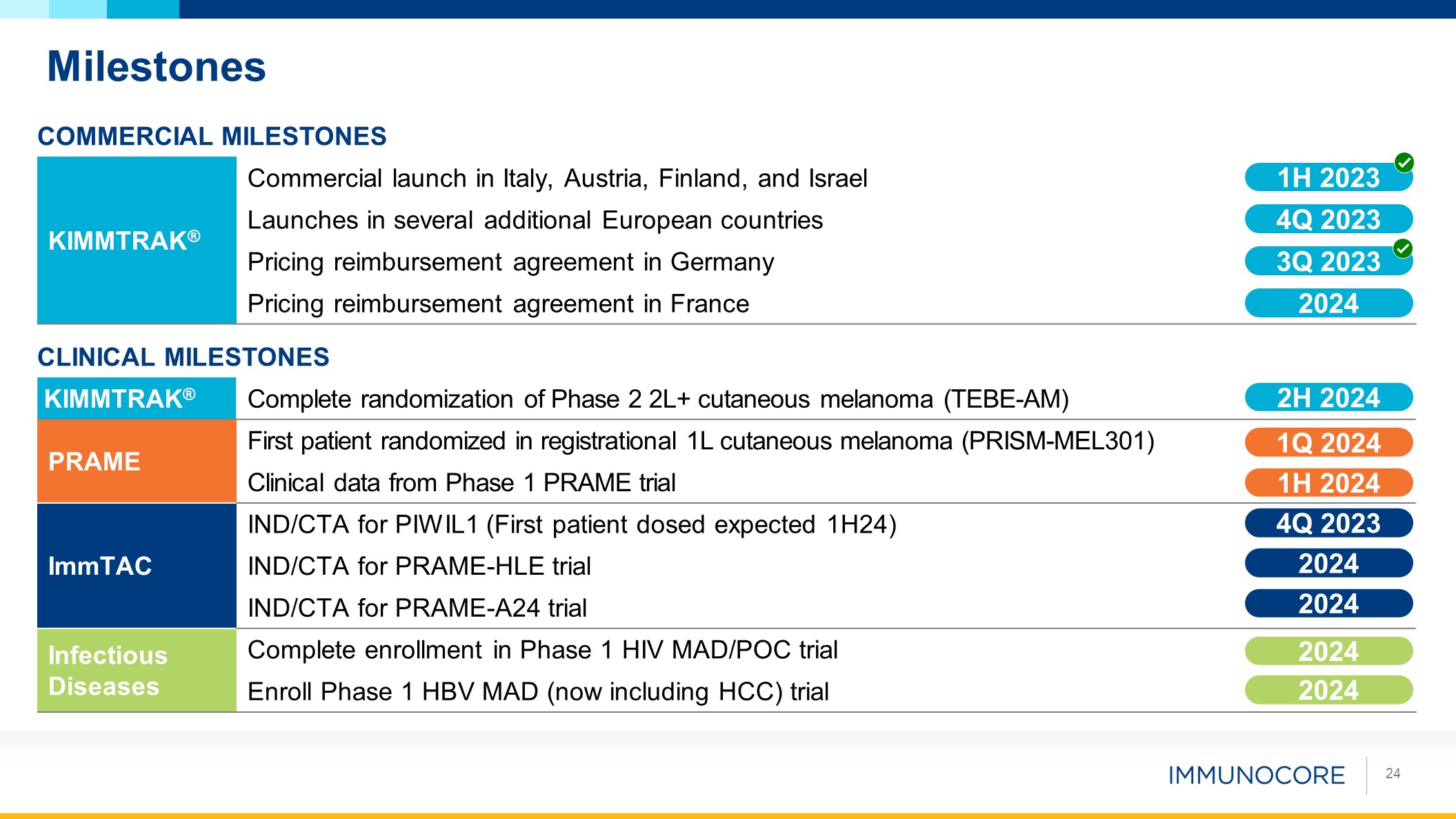
Commercial Milestones Planned Commercial Milestones KIMMTRAK® Commercial
launch in Italy, Austria, Finland, and Israel Launches in several additional European countries Pricing reimbursement agreement in Germany Pricing reimbursement agreement in France CLINICAL Milestones Planned Commercial
Milestones KIMMTRAK® Complete randomization of Phase 2 2L+ cutaneous melanoma (TEBE-AM) PRAME First patient randomized in registrational 1L cutaneous melanoma (PRISM-MEL301) Clinical data from Phase 1 PRAME trial ImmTAC IND/CTA
for PIWIL1 (First patient dosed expected 1H24) IND/CTA for PRAME-HLE trial IND/CTA for PRAME-A24 trial Infectious Diseases Complete enrollment in Phase 1 HIV MAD/POC trial Enroll Phase 1 HBV MAD (now including HCC) trial 1H
2023 4Q 2023 3Q 2023 2H 2024 1Q 2024 1H 2024 4Q 2023 2024 2024 2024 2024 Milestones 24 2024

BAHIJA JALLAL PhD Chief Executive Officer Q&A Session BRIAN DI
DONATO Chief Financial Officer and Head of Strategy DAVID BERMAN MD, PhD Head of Research and Development RALPH TORBAY Head of Commercial 25 MOHAMMED DAR MD SVP, Clinical Development and Chief Medical Officer

THANK YOU