Exhibit 99.2
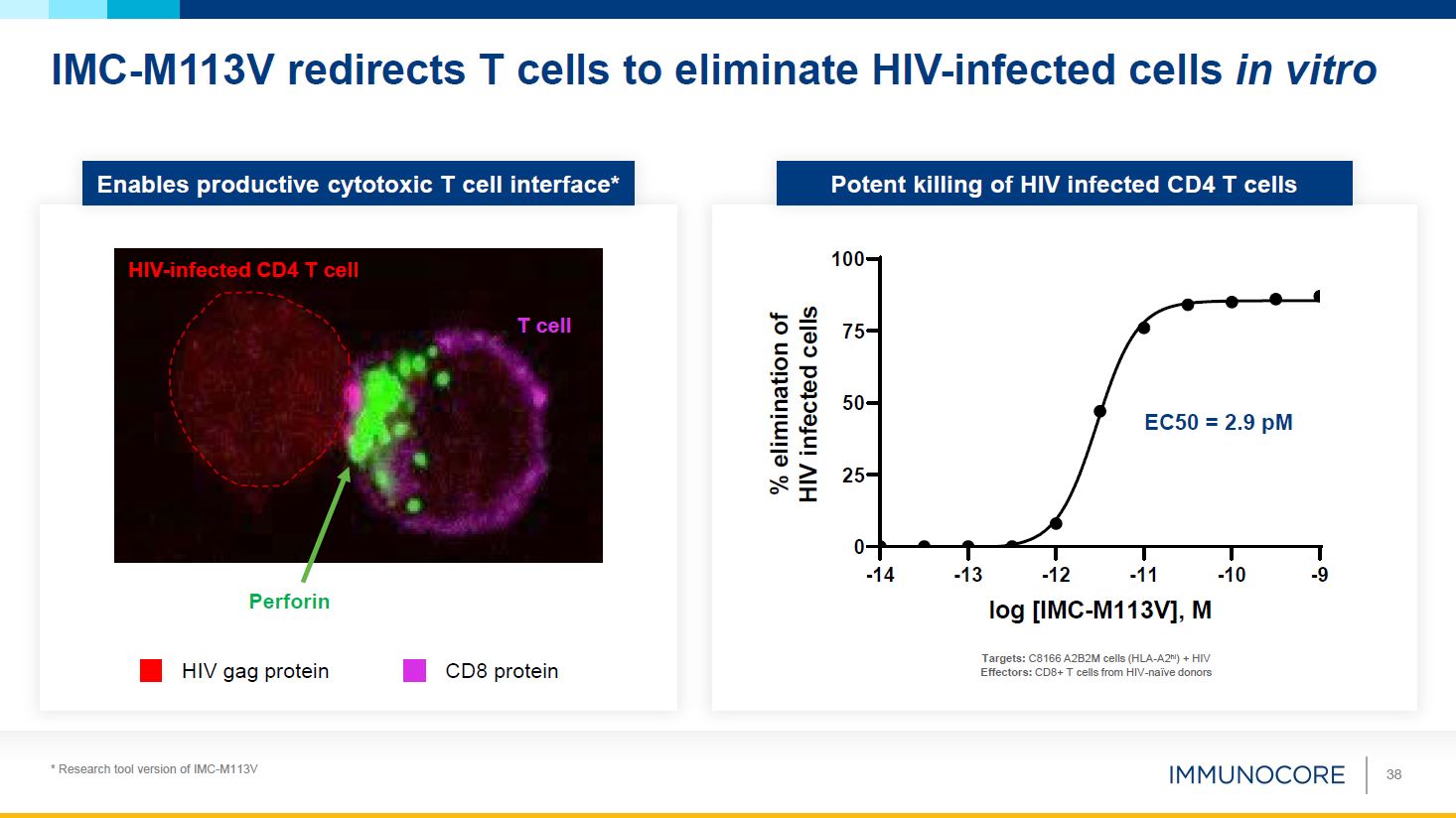
Enables productive cytotoxic T cell interface* Potent killing of HIV infected CD4
T cells IMC-M113V redirects T cells to eliminate HIV-infected cells in vitro * Research tool version of IMC-M113V 38 -14 -10 -9 25 0 50 75 100 -13 -12 -11 log [IMC-M113V], M Targets: C8166 A2B2M cells (HLA-A2hi) + HIV Effectors:
CD8+ T cells from HIV-naïve donors % elimination of HIV infected cells EC50 = 2.9 pM HIV gag protein CD8 protein T cell HIV-infected CD4 T cell Perforin
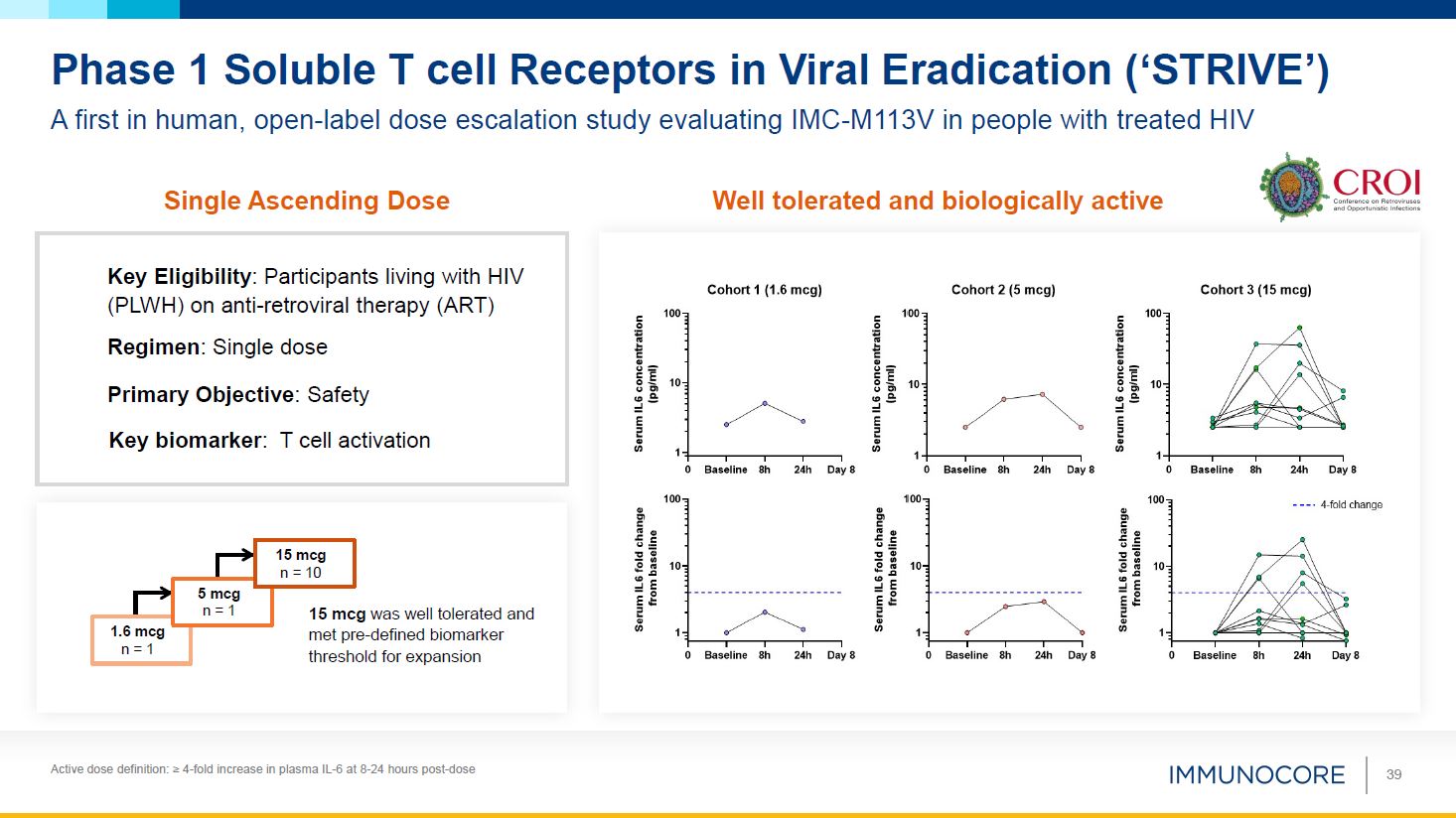
Active dose definition: ≥ 4-fold increase in plasma IL-6 at 8-24 hours
post-dose 39 Phase 1 Soluble T cell Receptors in Viral Eradication (‘STRIVE’) A first in human, open-label dose escalation study evaluating IMC-M113V in people with treated HIV Well tolerated and biologically active Single Ascending
Dose Key Eligibility: Participants living with HIV (PLWH) on anti-retroviral therapy (ART) Regimen: Single dose Primary Objective: Safety Key biomarker: T cell activation 1.6 mcg n = 1 5 mcg n = 1 15 mcg n = 10 15 mcg was well
tolerated and met pre-defined biomarker threshold for expansion
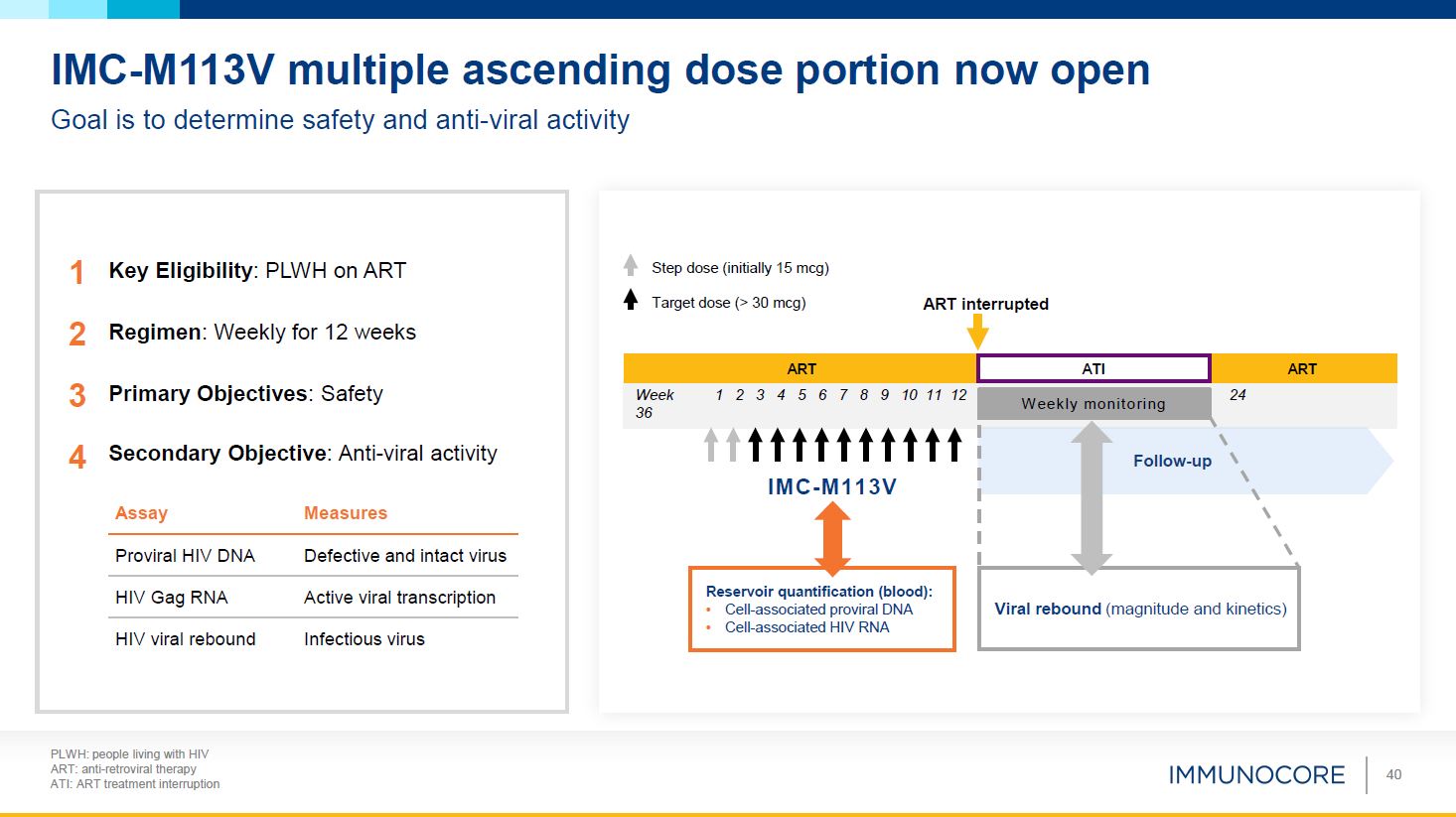
PLWH: people living with HIV ART: anti-retroviral therapy ATI: ART treatment
interruption 40 IMC-M113V multiple ascending dose portion now open Goal is to determine safety and anti-viral activity Key Eligibility: PLWH on ART Regimen: Weekly for 12 weeks Primary Objectives: Safety Viral rebound (magnitude and
kinetics) IMC-M113V 24 Week 1 2 3 4 5 6 7 8 9 10 11 12 36 ART ART ATI ART interrupted W eekly monitoring Step dose (initially 15 mcg) Target dose (> 30 mcg) Reservoir quantification (blood): Cell-associated proviral
DNA Cell-associated HIV RNA Follow-up Assay Measures Proviral HIV DNA Defective and intact virus HIV Gag RNA Active viral transcription HIV viral rebound Infectious virus 1 2 3 4 Secondary Objective: Anti-viral activity