
Transformative Medicines for Patients Bahija Jallal, PhD – Chief Executive Officer 41st
Annual J.P. Morgan Healthcare Conference JANUARY 11TH, 2023

This presentation contains forward-looking statements within the meaning of the
Private Securities Litigation Reform Act of 1995. Words such as “may,” “can,” “will,” “believe,” “expect,” “plan,” “anticipate”, “potential” and similar expressions (as well as other words or expressions referencing future events or
circumstances) are intended to identify forward-looking statements. All statements, other than statements of historical facts, included in this presentation are forward-looking statements. These statements include, but are not limited to,
statements regarding the marketing, therapeutic potential, and expected clinical benefits, including extended overall survival benefit and reduction in circulating tumor DNA, of Immunocore’s products and product candidates; expectations
regarding the development of Immunocore’s pipeline and the design, progress, timing, enrollment, scope, expansion and results of Immunocore’s existing, planned and other future clinical trials and IND enabling studies, including the targeted
delivery of IND for three new product candidates, the expansion of, and timing for reporting data from the monotherapy and combination arms of, the PRAME-A02 trial and the initiation of the multiple ascending dose portion of, and timing for
reporting data from the single ascending dose portion of, the IMC- M113V Phase 1 HIV clinical trial; the ability of TCR therapeutics to target approximately 90% of the human proteome; statements regarding the durability, efficacy and toleration
of Immunocore’s product candidates; expectations regarding the commercialization of KIMMTRAK including potential growth opportunities and trends and increasing access to KIMMTRAK; expectations regarding the value proposition of KIMMTRAK in
metastatic uveal melanoma (mUM) and advanced melanoma; expectations regarding the potential market size and opportunity for Immunocore’s products and product candidates, including statements with respect to potential patient population;
expectations regarding the number of patients that PRAME-A02 has the potential to benefit; statements that IMC-R117C is a first-in-class PIWIL1-targeted immunotherapy under development; statements regarding the planned IND timing for IMC-R117C;
expectations regarding future milestones; future development plans of tebentafusp and Immunocore’s other product candidates; the ability to obtain and maintain regulatory approval for its products and product candidates; expectations regarding
the sustained or potential commercial performance and uptake of KIMMTRAK and Immunocore’s other product candidates, if approved; expectations regarding Immunocore’s management of resources and expected cash runway; and preliminary unaudited net
sales and cash and cash equivalents of KIMMTRAK and tebentafusp; and the validation of the ImmTAC platform. These forward-looking statements are based on management’s current expectations and beliefs and are subject to a number of risks,
uncertainties and important factors that may cause actual events or results to differ materially and adversely from those expressed or implied by any forward-looking statements, many of which are beyond Immunocore’s control. These include,
without limitation, risks and uncertainties related to the impact of worsening macroeconomic conditions and the ongoing and evolving COVID-19 pandemic, the war in Ukraine or global geopolitical tension on Immunocore’s business, strategy,
clinical trials, financial position and anticipated milestones, including Immunocore’s ability to conduct ongoing and planned clinical trials; Immunocore’s ability to obtain and maintain regulatory approval of its product candidates;
Immunocore’s ability to obtain clinical supply of current or future product candidates or commercial supply of KIMMTRAK or any future approved products, including as a result of supply chain disruptions; Immunocore’s ability to develop,
manufacture and commercialize its product candidates; Immunocore’s ability and plans to launch, market and sell KIMMTRAK or any future approved products, to continue to establish and expand a commercial infrastructure; Immunocore’s ability to
successfully expand the approved indications for KIMMTRAK, or obtain marketing approval for KIMMTRAK in additional geographies in the future; the delay of any current or planned clinical trials, whether due to the COVID- 19 pandemic, patient
enrollment delays or otherwise; unexpected safety or efficacy data observed during preclinical studies or clinical trials and Immunocore’s ability to successfully demonstrate the safety and efficacy of its product candidates and gain approval
of its product candidates on a timely basis, if at all; competition with respect to market opportunities; actions of regulatory agencies, which may affect the initiation, timing and progress of clinical trials or future regulatory approval;
Immunocore’s ability to obtain, maintain and enforce intellectual property protection for KIMMTRAK or any product candidates it is developing; clinical trial site activation or enrollment rates that are lower than expected; Immunocore’s need
for and ability to obtain additional funding on favorable terms or at all, including as a result of worsening macroeconomic conditions such as rising inflation and interest rates, volatility in the capital markets and related market
uncertainty; and the success of Immunocore’s current and future collaborations, partnerships or licensing arrangements. These and other risks and uncertainties are described in greater detail in the section titled ‘Risk Factors’; in
Immunocore’s filings with the Securities and Exchange Commission, including Immunocore’s most recent Annual Report on Form 20-F, as supplemented by its most recent filings that Immunocore has made or may make with the SEC in the future. Such
risks may be amplified by the COVID-19 pandemic and its potential impact on Immunocore’s business and the overall global economy. Any forward-looking statements represent Immunocore’s views only as of the date of this presentation and should
not be relied upon as representing its views as of any subsequent date. Immunocore does not assume any obligation to update any forward-looking statements, except as may be required by law. In addition, as the reported net sales and cash and
cash equivalents in this presentation are preliminary, have not been audited and are subject to change pending completion of our audited financial statements for the year ended December 31, 2022, it is possible that Immunocore or its
independent registered public accounting firm may identify items that require Immunocore to make adjustments to the amount included in this presentation, and such changes could be material. Additional information and disclosures would also be
required for a more complete understanding of Immunocore’s financial position and results of operations as of December 31, 2022. Certain information contained in this presentation relates to or is based on studies, publications, surveys, and
other data obtained from third-party sources and Immunocore’s own internal estimates and research. While Immunocore believes these third-party sources to be reliable as of the date of this presentation, it has not independently verified, and
makes no representation as to the adequacy, fairness, accuracy, or completeness of, any information obtained from third-party sources. KIMMTRAK™ is a trademark owned or licensed to Immunocore. Forward-looking statement 2
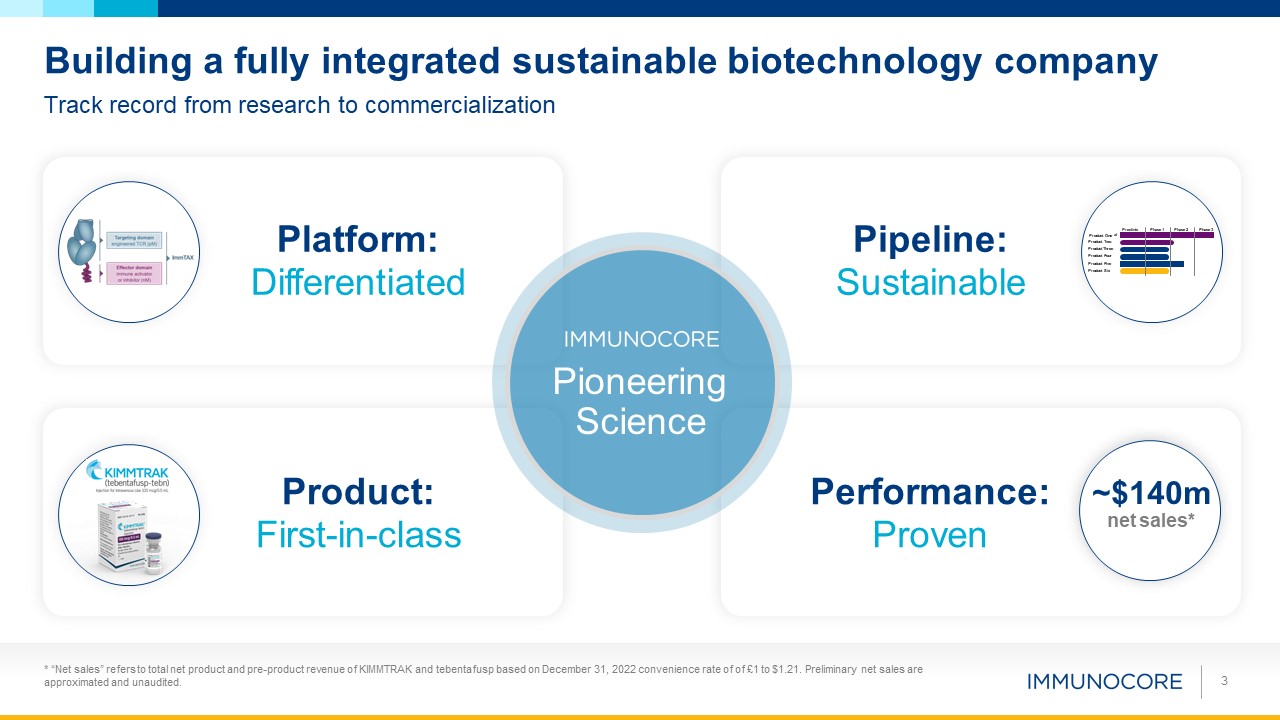
Platform: Differentiated Building a fully integrated sustainable biotechnology
company Track record from research to commercialization * “Net sales” refers to total net product and pre-product revenue of KIMMTRAK and tebentafusp based on December 31, 2022 convenience rate of of £1 to $1.21. Preliminary net sales are
approximated and unaudited. 3 Product Two Product Three Product Four Product Five Product Six Preclinic Product One al Phase 1 Phase 2 Phase 3 Pipeline: Sustainable Performance: Proven ~$140m net
sales* Product: First-in-class Pioneering Science

Our mission To radically improve outcomes for patients with cancer, infectious diseases,
and autoimmune conditions by pioneering and delivering transformative medicines 4
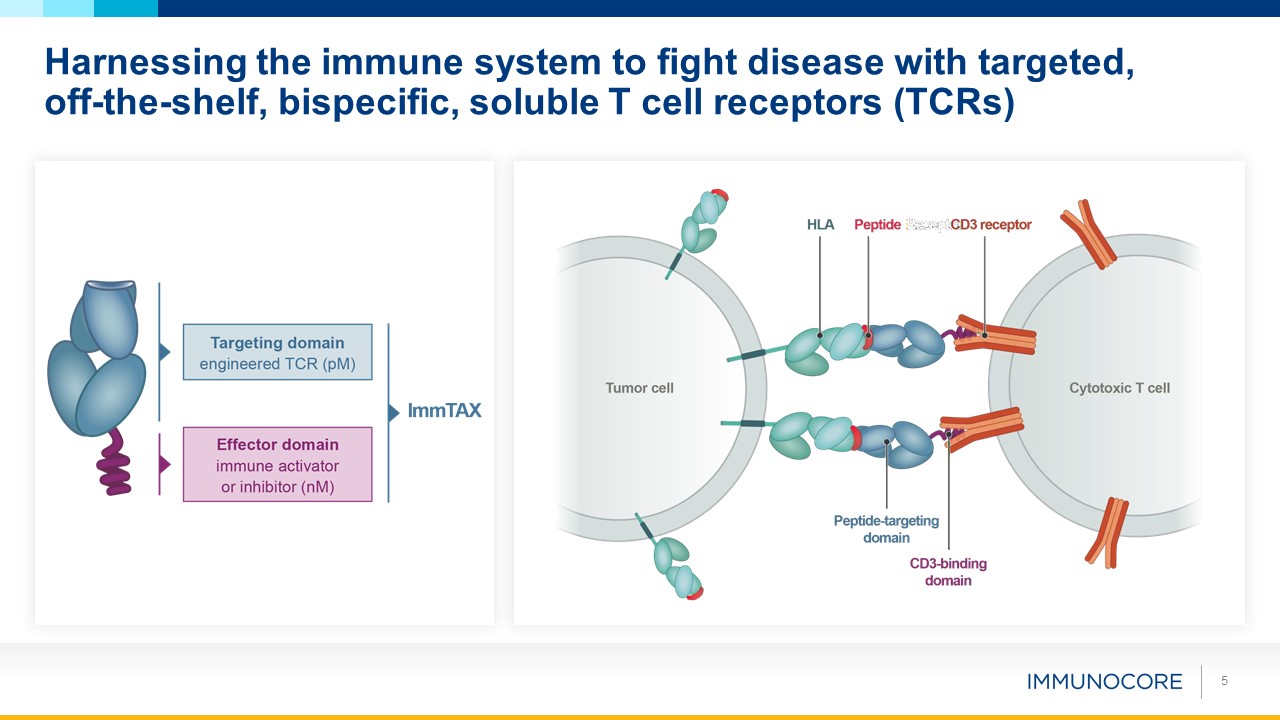
5 Harnessing the immune system to fight disease with targeted, off-the-shelf, bispecific,
soluble T cell receptors (TCRs)
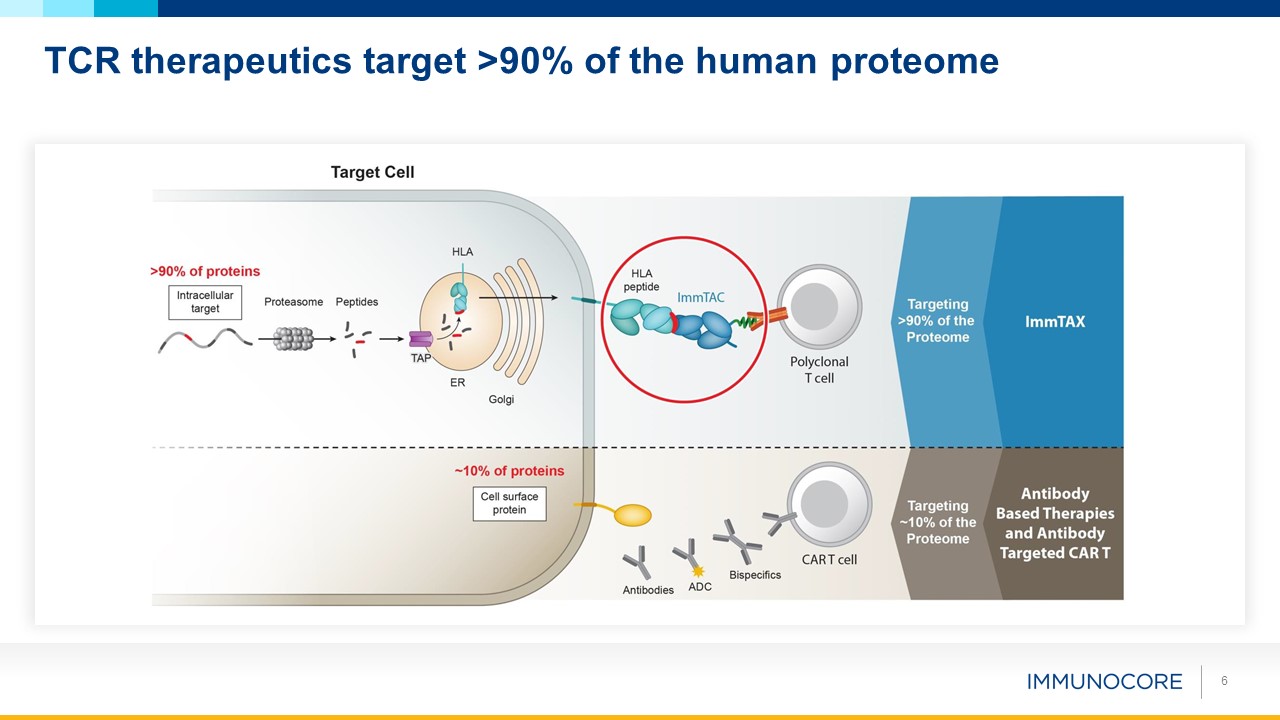
6 TCR therapeutics target >90% of the human proteome
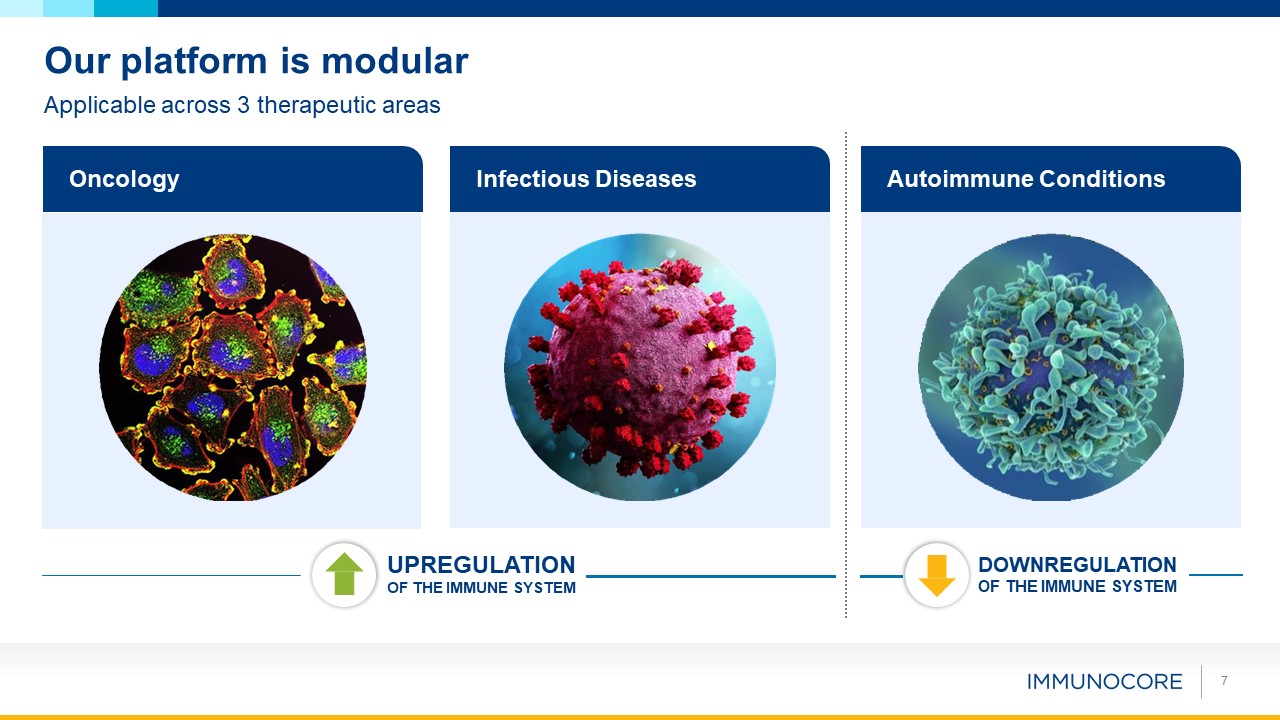
Our platform is modular Applicable across 3 therapeutic areas 7 Oncology Infectious
Diseases Autoimmune Conditions UPREGULATION OF THE IMMUNE SYSTEM DOWNREGULATION OF THE IMMUNE SYSTEM
Today’s presentation will cover oncology and infectious diseases Infectious
Diseases 8 Oncology UPREGULATION OF THE IMMUNE SYSTEM Autoimmune Conditions DOWNREGULATION OF THE IMMUNE SYSTEM

The next chapter in oncology

We have written the next chapter in cancer treatment 10 T Cell Receptor (TCR)
Therapy Off-the-shelf bispecific T cell engagers 1949 Targeted Therapy Chemotherapy 1997 Immunotherapy 2011 Antibody-Drug Conjugate 2013 Cell Therapy 2017 2022

KIMMTRAK® 11
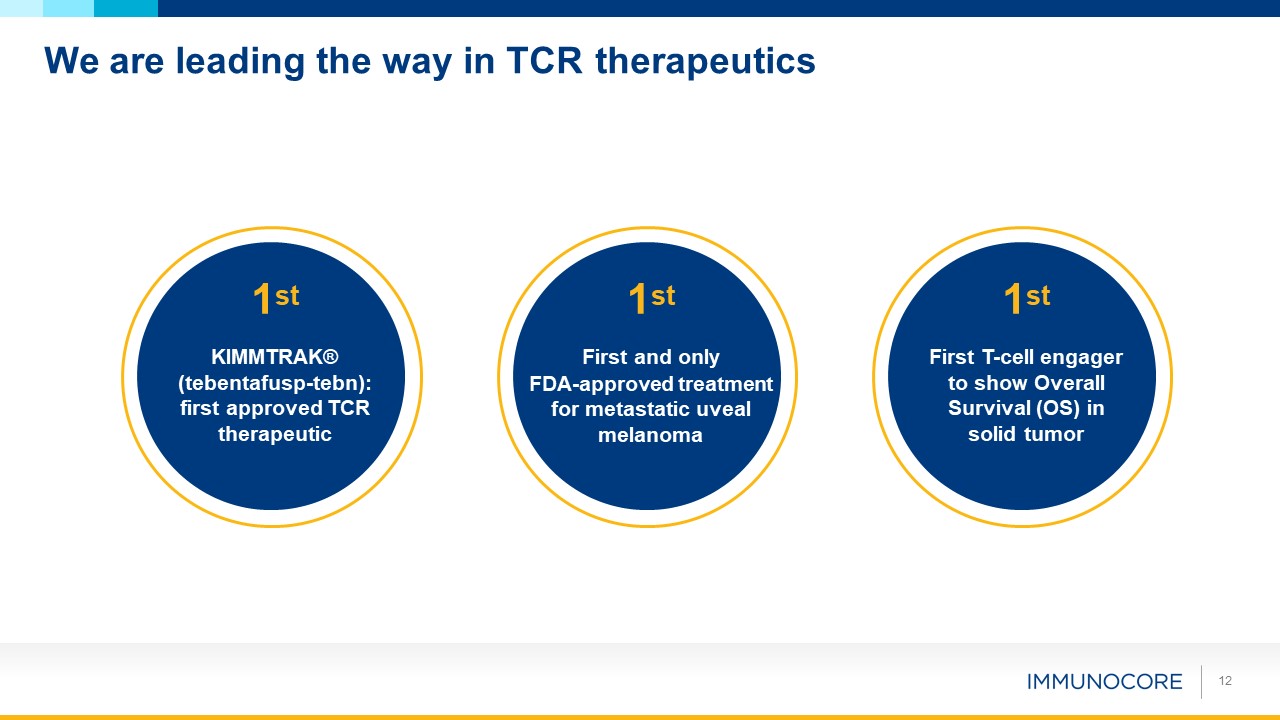
12 We are leading the way in TCR therapeutics 1st First and only FDA-approved treatment
for metastatic uveal melanoma 1st KIMMTRAK® (tebentafusp-tebn): first approved TCR therapeutic 1st First T-cell engager to show Overall Survival (OS) in solid tumor
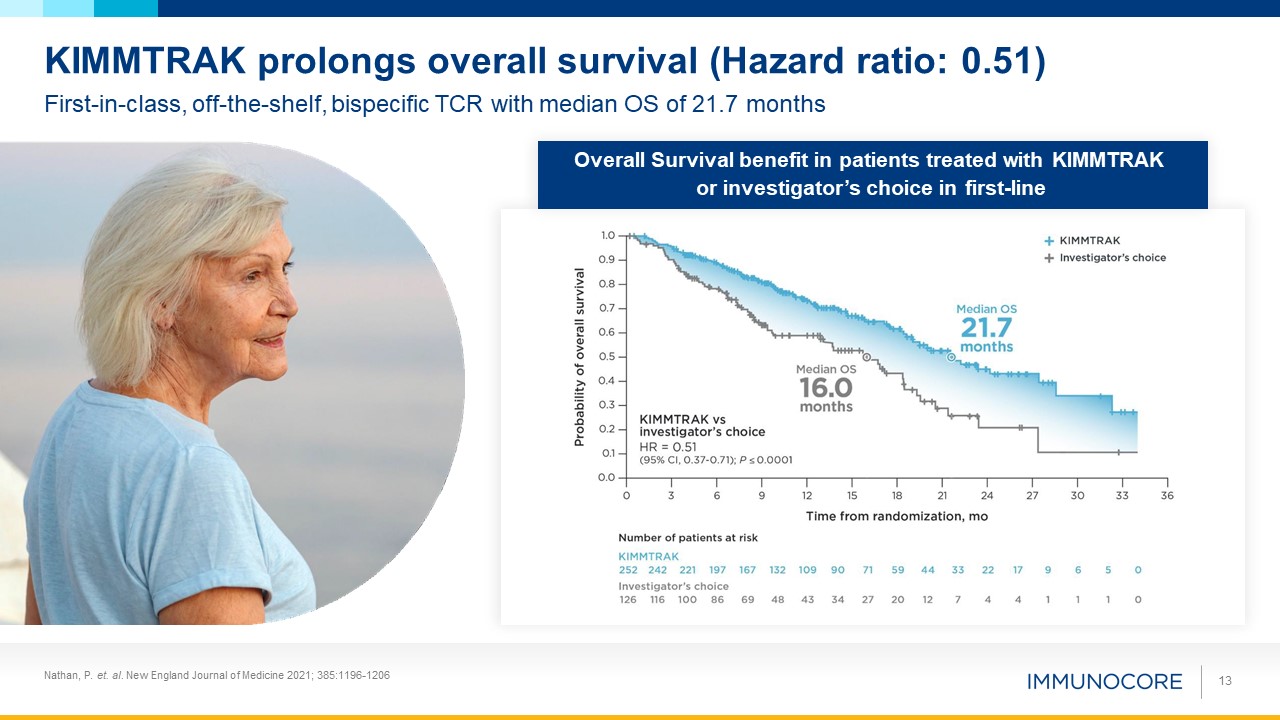
Nathan, P. et. al. New England Journal of Medicine 2021; 385:1196-1206 13 KIMMTRAK
prolongs overall survival (Hazard ratio: 0.51) First-in-class, off-the-shelf, bispecific TCR with median OS of 21.7 months Overall Survival benefit in patients treated with KIMMTRAK or investigator’s choice in first-line
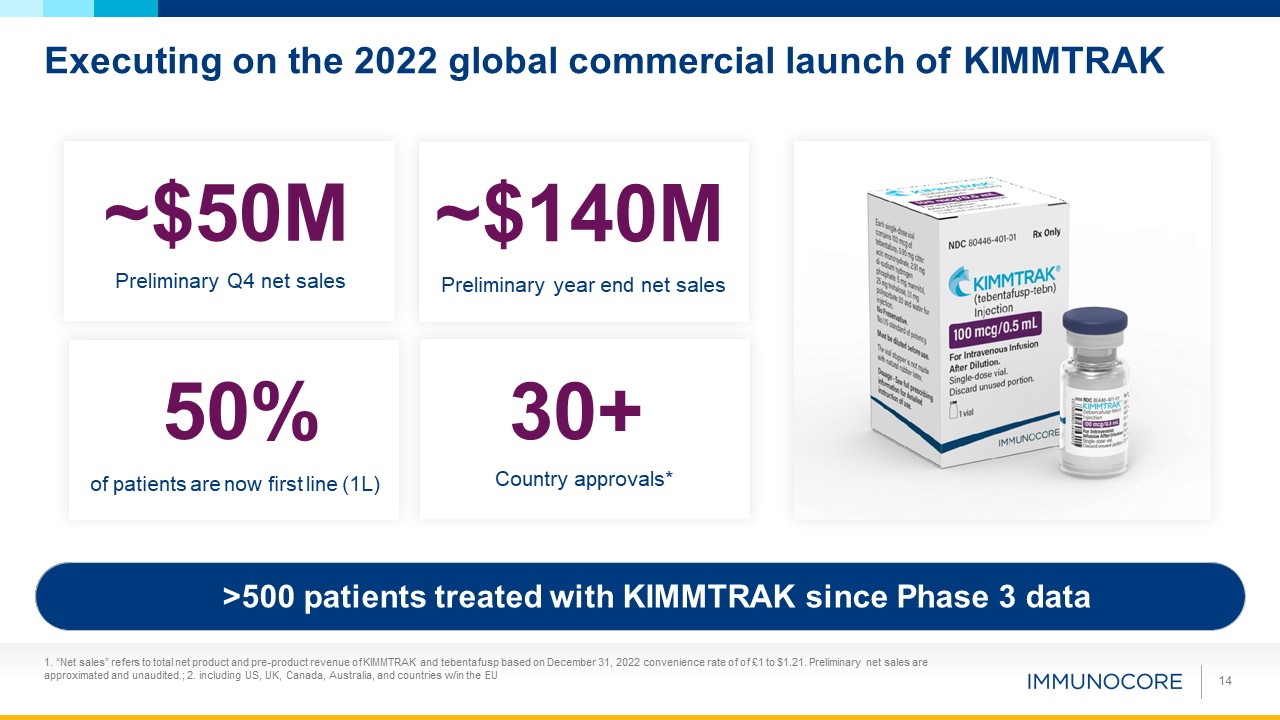
Executing on the 2022 global commercial launch of KIMMTRAK 14 ~$50M Preliminary Q4 net
sales 30+ Country approvals* 50% of patients are now first line (1L) ~$140M Preliminary year end net sales >500 patients treated with KIMMTRAK since Phase 3 data 1. “Net sales” refers to total net product and pre-product revenue of
KIMMTRAK and tebentafusp based on December 31, 2022 convenience rate of of £1 to $1.21. Preliminary net sales are approximated and unaudited.; 2. including US, UK, Canada, Australia, and countries w/in the EU
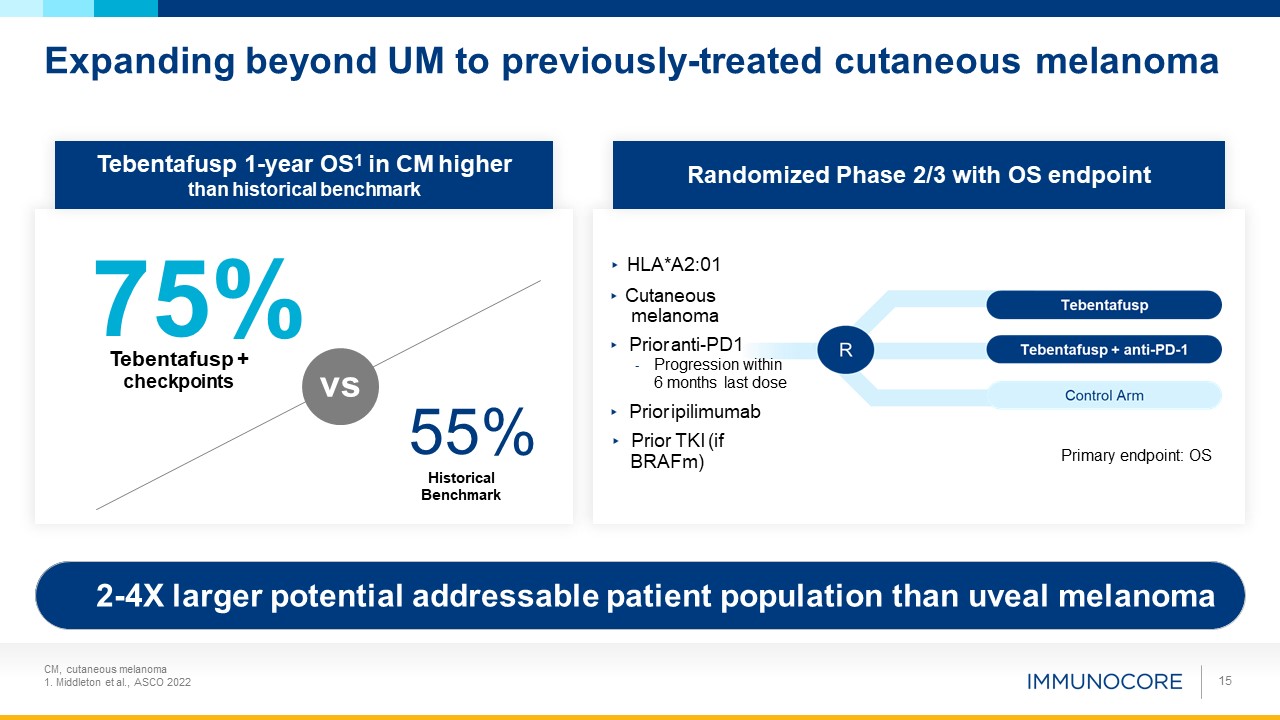
Expanding beyond UM to previously-treated cutaneous melanoma 15 Tebentafusp 1-year OS1 in
CM higher than historical benchmark 75% Tebentafusp + checkpoints 55% Historical Benchmark Randomized Phase 2/3 with OS endpoint Primary endpoint: OS vs 2-4X larger potential addressable patient population than uveal melanoma ▸
HLA*A2:01 ▸ Cutaneous melanoma ▸ Prior anti-PD1 - Progression within 6 months last dose ▸ Prior ipilimumab ▸ Prior TKI (if BRAFm) CM, cutaneous melanoma 1. Middleton et al., ASCO 2022

PRAME Franchise: A02, A24, A02-HLE
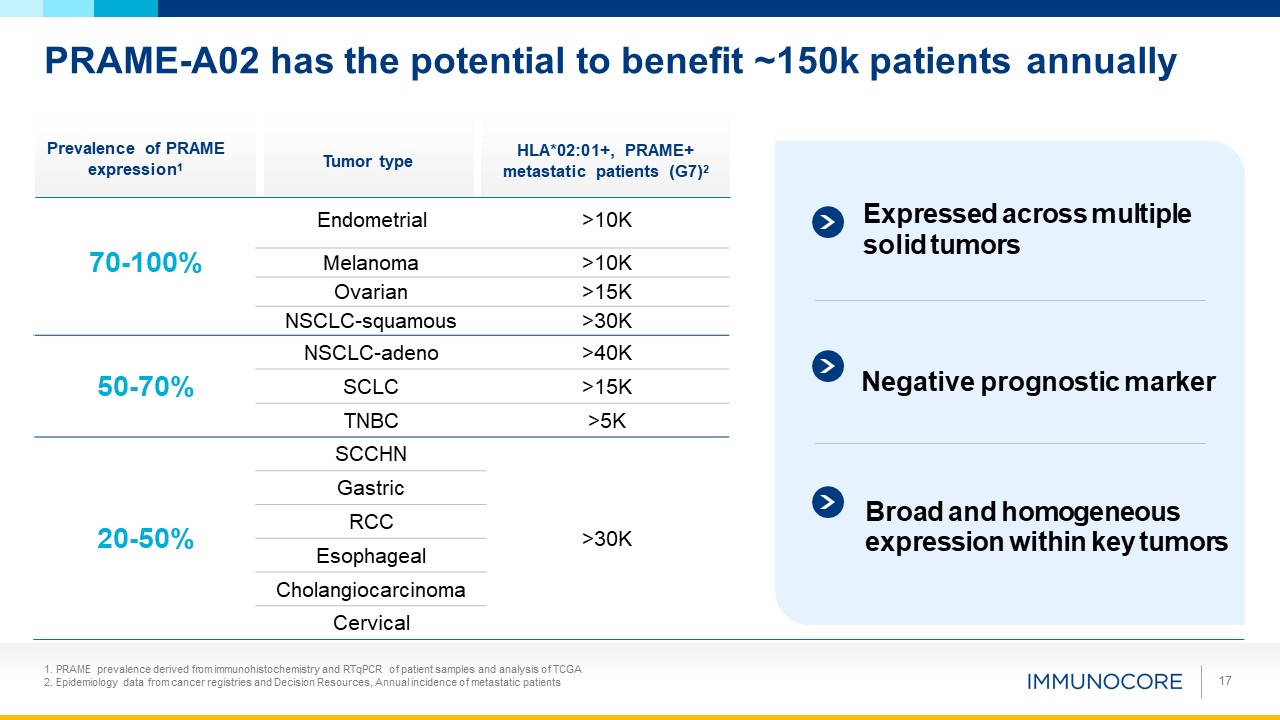
PRAME-A02 has the potential to benefit ~150k patients annually Tumor type Prevalence of
PRAME expression1 HLA*02:01+, PRAME+ metastatic patients (G7)2 17 PRAME prevalence derived from immunohistochemistry and RTqPCR of patient samples and analysis of TCGA Epidemiology data from cancer registries and Decision Resources, Annual
incidence of metastatic patients Endometrial >10K Expressed across multiple 70-100% solid tumors Melanoma >10K Ovarian >15K NSCLC-squamous >30K 50-70% NSCLC-adeno >40K Negative prognostic
marker SCLC >15K TNBC >5K SCCHN Gastric Broad and homogeneous 20-50% RCC >30K expression within key tumors Esophageal Cholangiocarcinoma Cervical
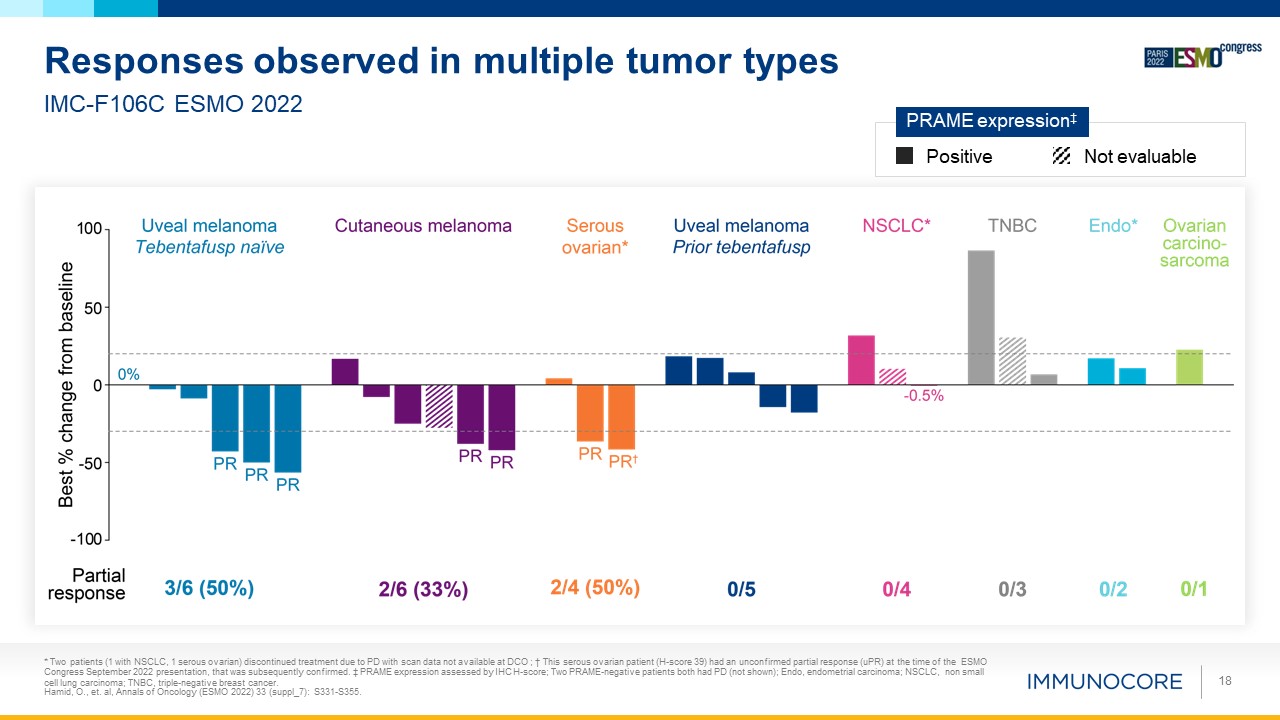
* Two patients (1 with NSCLC, 1 serous ovarian) discontinued treatment due to PD with scan
data not available at DCO ; † This serous ovarian patient (H-score 39) had an unconfirmed partial response (uPR) at the time of the ESMO Congress September 2022 presentation, that was subsequently confirmed. ‡ PRAME expression assessed by IHC
H-score; Two PRAME-negative patients both had PD (not shown); Endo, endometrial carcinoma; NSCLC, non small cell lung carcinoma; TNBC, triple-negative breast cancer. Hamid, O., et. al, Annals of Oncology (ESMO 2022) 33 (suppl_7):
S331-S355. 18 Responses observed in multiple tumor types IMC-F106C ESMO 2022 Positive Not evaluable PRAME expression‡
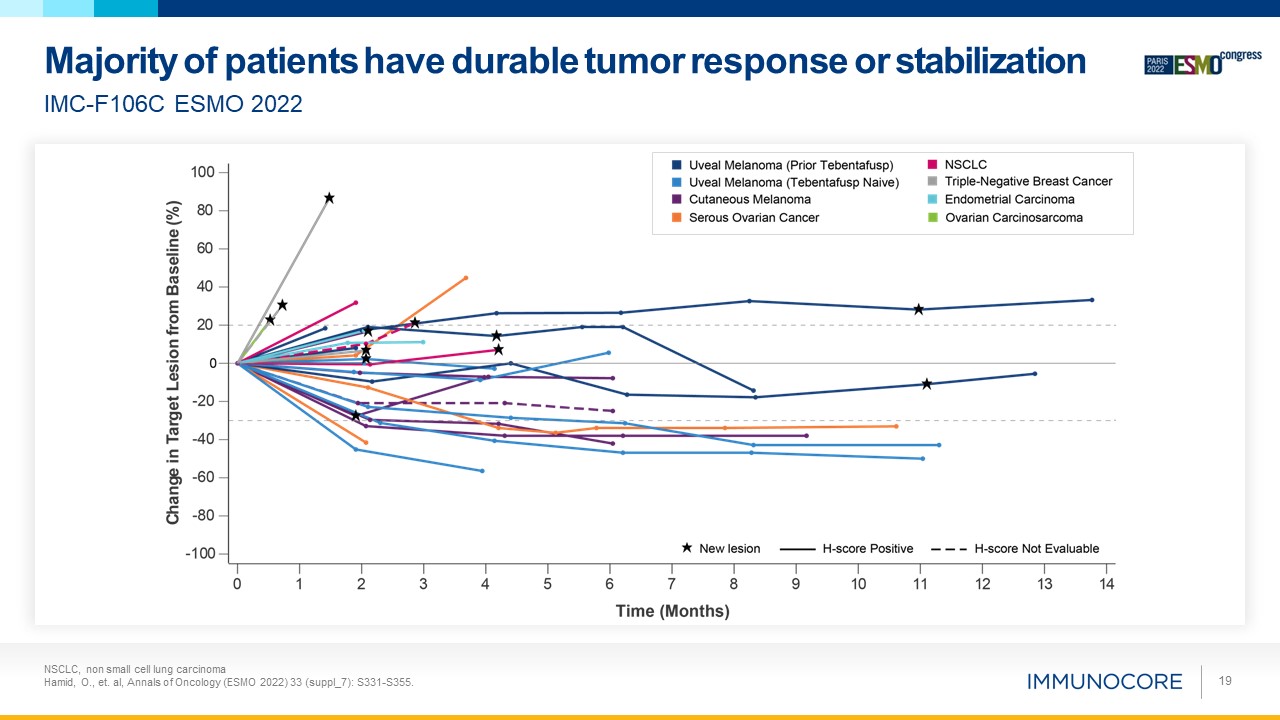
NSCLC, non small cell lung carcinoma Hamid, O., et. al, Annals of Oncology (ESMO 2022) 33
(suppl_7): S331-S355. 19 Majority of patients have durable tumor response or stabilization IMC-F106C ESMO 2022
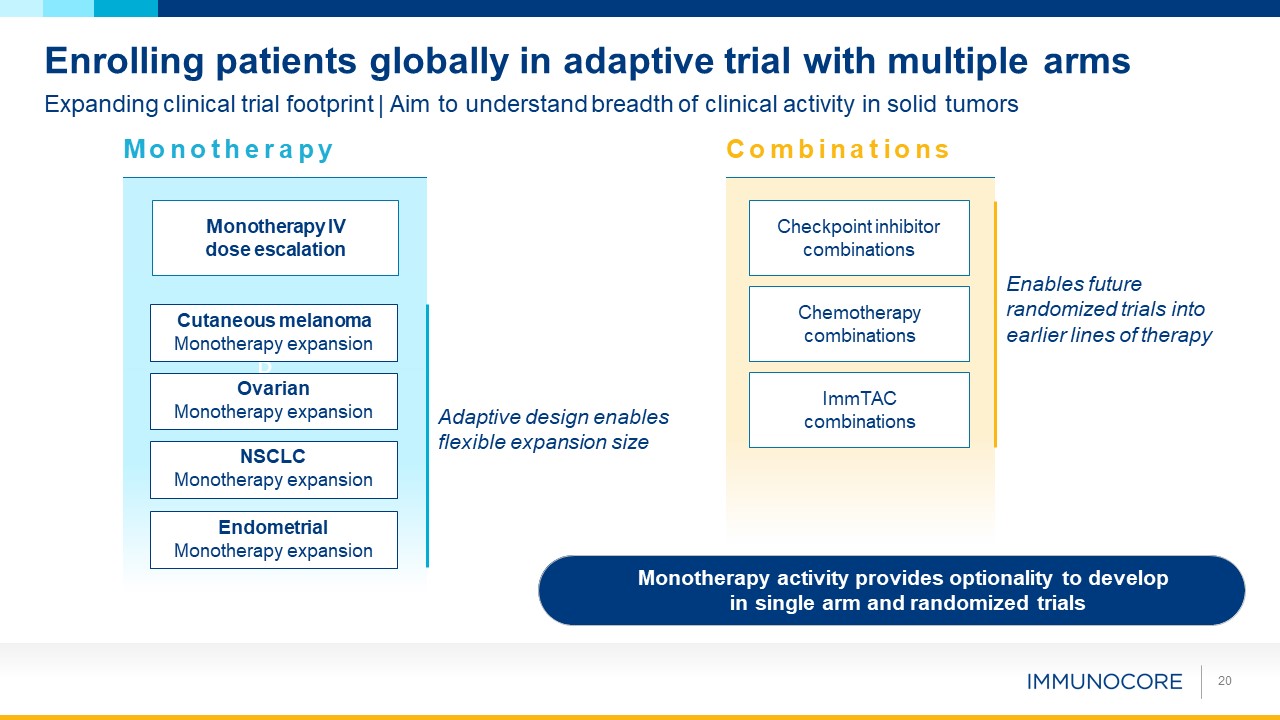
Monotherapy activity provides optionality to develop in single arm and randomized
trials 20 Enrolling patients globally in adaptive trial with multiple arms P Endometrial Monotherapy expansion Monotherapy IV dose escalation Checkpoint inhibitor combinations ImmTAC combinations Chemotherapy combinations Expanding
clinical trial footprint | Aim to understand breadth of clinical activity in solid tumors M o n o t h e r a p y C o m b i n a t i o n s NSCLC Monotherapy expansion Ovarian Monotherapy expansion Cutaneous melanoma Monotherapy
expansion Enables future randomized trials into earlier lines of therapy Adaptive design enables flexible expansion size
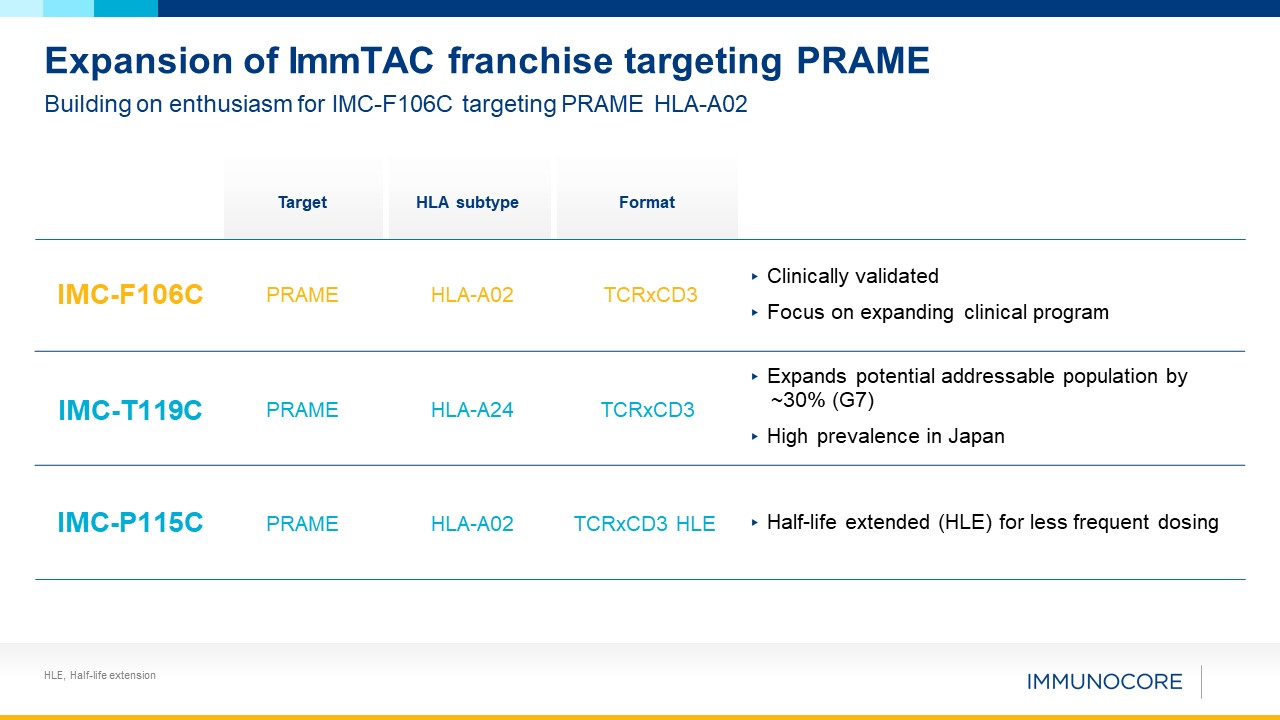
HLE, Half-life extension Expansion of ImmTAC franchise targeting PRAME Building on
enthusiasm for IMC-F106C targeting PRAME HLA-A02 IMC-F106C PRAME HLA-A02 TCRxCD3 ▸ Clinically validated ▸ Focus on expanding clinical program IMC-T119C PRAME HLA-A24 TCRxCD3 ▸ Expands potential addressable population by ~30% (G7) ▸
High prevalence in Japan IMC-P115C PRAME HLA-A02 TCRxCD3 HLE ▸ Half-life extended (HLE) for less frequent dosing Target HLA subtype Format

Novel ImmTAC Candidate for GI cancers from our discovery engine
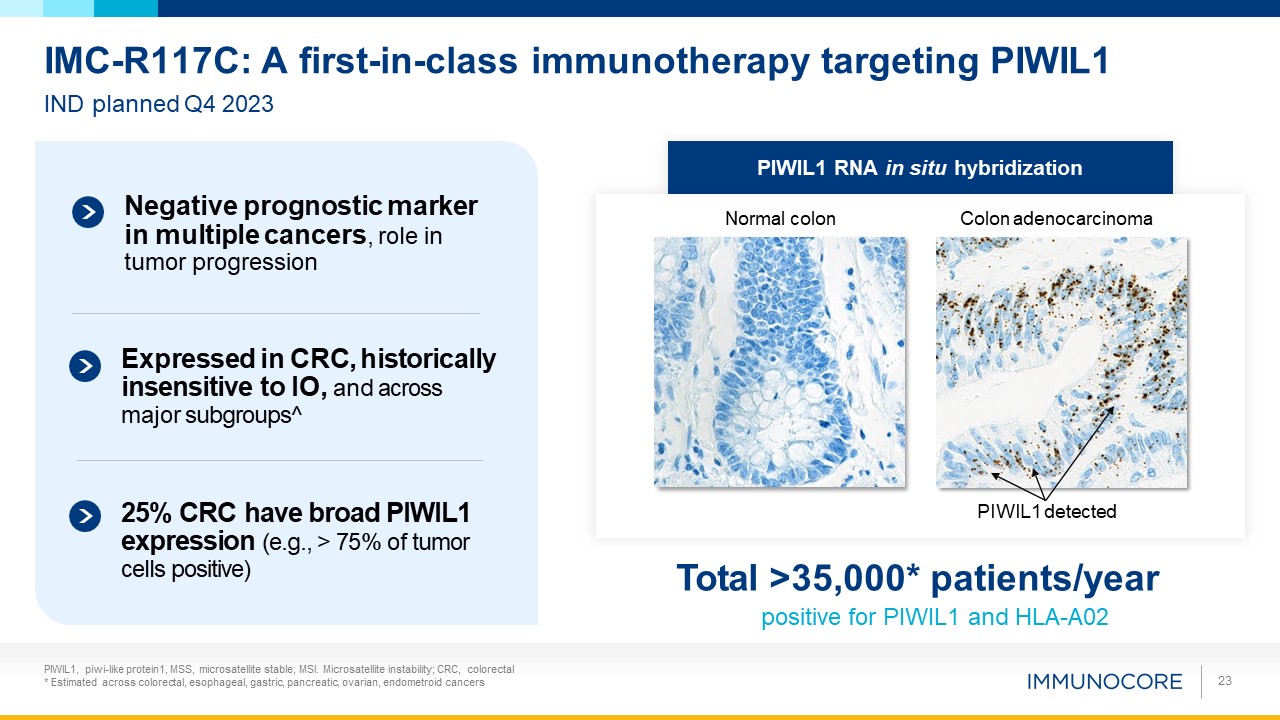
PIWIL1, piwi-like protein1, MSS, microsatellite stable; MSI. Microsatellite instability;
CRC, colorectal * Estimated across colorectal, esophageal, gastric, pancreatic, ovarian, endometroid cancers 23 IMC-R117C: A first-in-class immunotherapy targeting PIWIL1 IND planned Q4 2023 Total >35,000* patients/year positive for
PIWIL1 and HLA-A02 PIWIL1 RNA in situ hybridization Normal colon Colon adenocarcinoma PIWIL1 detected Negative prognostic marker in multiple cancers, role in tumor progression Expressed in CRC, historically insensitive to IO, and across
major subgroups^ 25% CRC have broad PIWIL1 expression (e.g., > 75% of tumor cells positive)

Pursuing a functional cure in infectious diseases
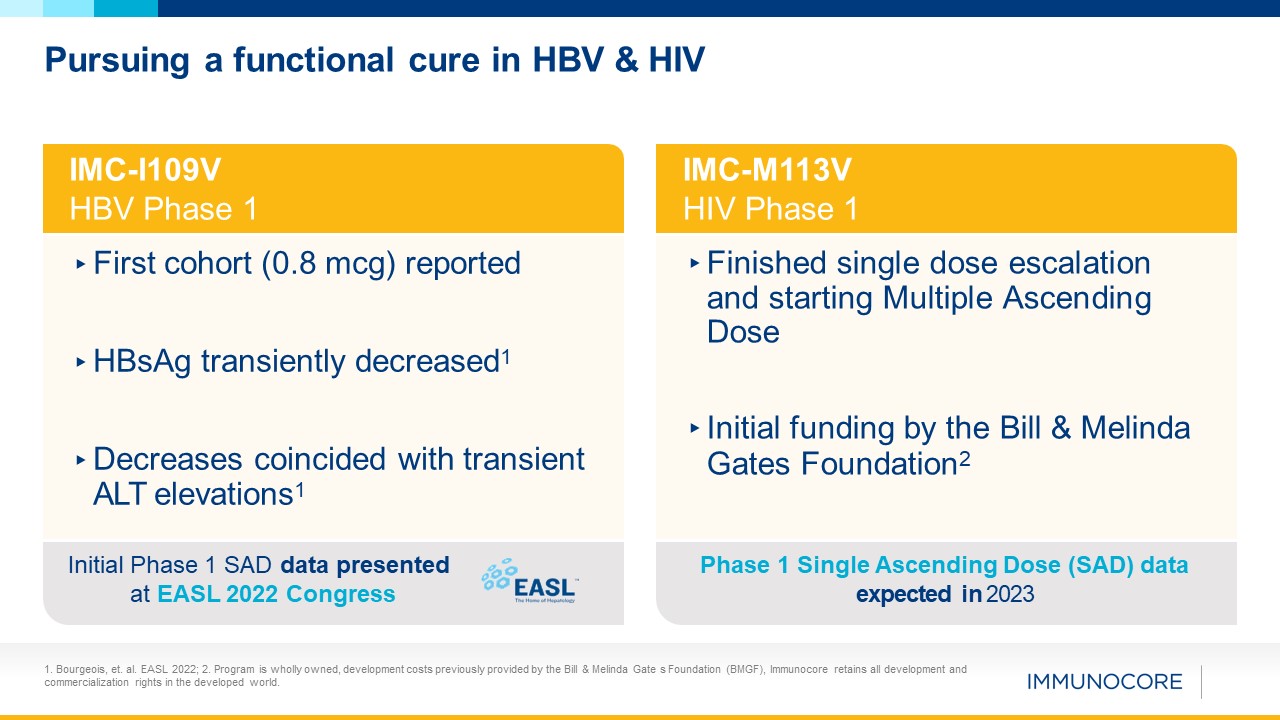
Pursuing a functional cure in HBV & HIV IMC-I109V HBV Phase 1 ▸First cohort (0.8
mcg) reported ▸HBsAg transiently decreased1 ▸Decreases coincided with transient ALT elevations1 Initial Phase 1 SAD data presented at EASL 2022 Congress IMC-M113V HIV Phase 1 ▸Finished single dose escalation and starting Multiple
Ascending Dose ▸Initial funding by the Bill & Melinda Gates Foundation2 Phase 1 Single Ascending Dose (SAD) data expected in 2023 1. Bourgeois, et. al. EASL 2022; 2. Program is wholly owned, development costs previously provided by the
Bill & Melinda Gate s Foundation (BMGF), Immunocore retains all development and commercialization rights in the developed world.

Delivering on our promise – Consistent execution
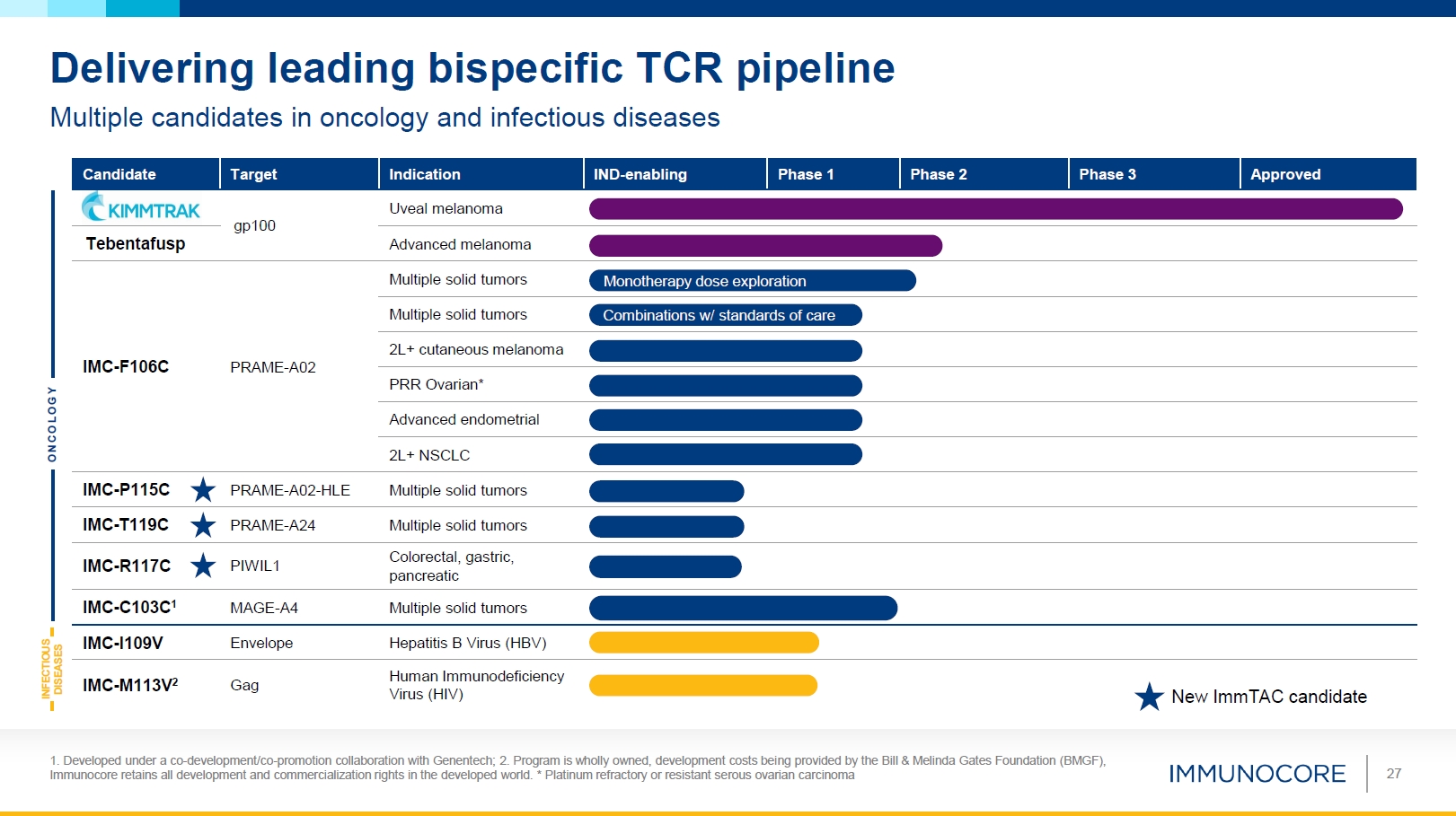
27 Delivering leading bispecific TCR pipeline Multiple candidates in oncology and
infectious diseases O N C O L O G Y INFECTIOUS DISEASES Candidate Target Indication IND-enabling Phase 1 Phase 2 Phase 3 Approved gp100 Uveal melanoma Advanced melanoma Tebentafusp Multiple solid tumors Monotherapy dose
explor ation Multiple solid tumors Combinations w/ standar ds of care IMC-F106C PRAME-A02 2L+ cutaneous melanoma PRR Ovarian* Advanced endometrial 2L+ NSCLC IMC-P115C PRAME-A02-HLE Multiple solid
tumors IMC-T119C PRAME-A24 Multiple solid tumors IMC-R117C PIWIL1 Colorectal, gastric, pancreatic IMC-C103C1 MAGE-A4 Multiple solid tumors IMC-I109V Envelope Hepatitis B Virus (HBV) IMC-M113V2 Gag Human Immunodeficiency Virus
(HIV) New Im mTAC candidate 1. Developed under a co-development/co-promotion collaboration with Genentech; 2. Program is wholly owned, development costs being provided by the Bill & Melinda Gates Foundation (BMGF), Immunocore retains all
development and commercialization rights in the developed world. * Platinum refractory or resistant serous ovarian carcinoma

~$400M *Preliminary financial results are approximated and unaudited. 1. “Net sales”
refers to total net product and net pre-product revenue of KIMMTRAK and tebentafusp. 2. Dollar amounts based on conversion rate of approximately 1.21. 28 Q4 preliminary net sales of KIMMTRAK / tebentafusp1,2 Preliminary cash and
cash equivalents as of December 31, 20222 YE preliminary net sales of KIMMTRAK / tebentafusp1,2 ~$50M ~$140M Preliminary 2022 Financial Results Cash runway projected into 2026 with anticipated KIMMTRAK revenues

41st Annual J.P. Morgan Healthcare Conference 29 Looking ahead Continuing to write the
next chapter of cancer and infectious diseases treatment Sustain and grow Global site expansion for PRAME-A02 trial (data by 1H 2024) Deliver IND for 3 new ImmTAC candidates HIV Phase 1 SAD data 2023 Continue responsible management of
resources

Experienced team with deep scientific & commercial expertise 30 Andy Hooker VP, CMC
& Supply Chain JoAnn Suzich Head of Research Ralph Torbay Head of Commercial Mark Moyer Head of Regulatory CIMZIA SYNAGIS, FLUMIST, VLP technology for HPV vaccines YERVOY, OPDIVO, TAXOTERE, ZOLADEX, PLAVIX, JEVTANA,
ELOXATIN IMFINZI, TAGRISSO, CALQUENCE, GLEEVEC, TASIGNA, ARZERRA, FARYDAK Bahija Jallal CEO Brian Di Donato CFO & Head of Strategy Mohammed Dar CMO David Berman Head of R&D YERVOY, EMPLICITI, LUMOXITI, IMFINZI VOTRIENT,
IMFINZI, LUMOXITI IMFINZI, FASENRA, LUMOXITI, SELIQ, QAIV, SAPHNELO Regulatory approval of KIMMTRAK® in unresectable or metastatic uveal melanoma (mUM) in 30+ countries

THANK YOU
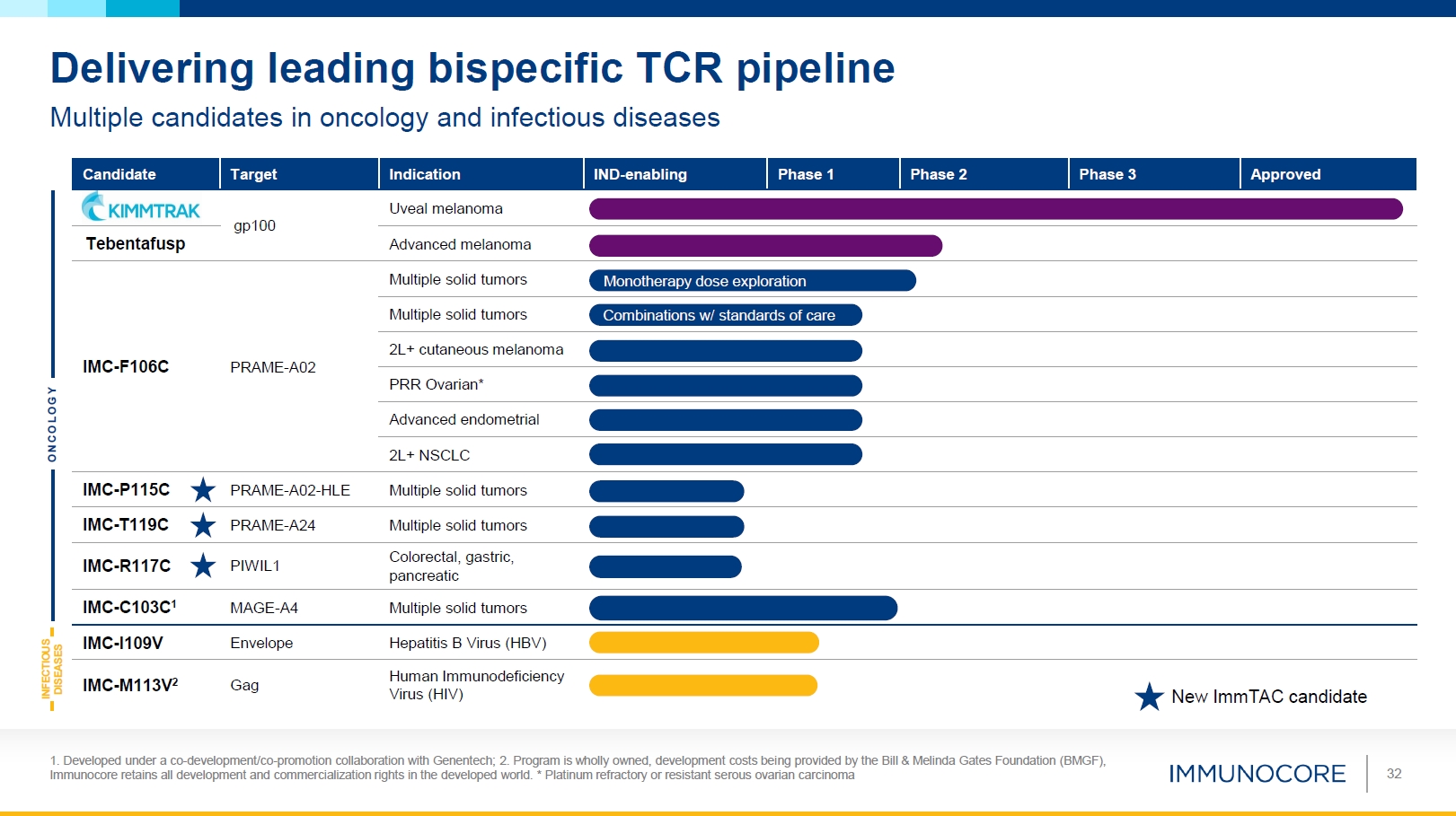
32 Delivering leading bispecific TCR pipeline Multiple candidates in oncology and
infectious diseases O N C O L O G Y INFECTIOUS DISEASES Candidate Target Indication IND-enabling Phase 1 Phase 2 Phase 3 Approved gp100 Uveal melanoma Advanced melanoma Tebentafusp Multiple solid tumors Monotherapy dose
explor ation Multiple solid tumors Combinations w/ standar ds of care IMC-F106C PRAME-A02 2L+ cutaneous melanoma PRR Ovarian* Advanced endometrial 2L+ NSCLC IMC-P115C PRAME-A02-HLE Multiple solid
tumors IMC-T119C PRAME-A24 Multiple solid tumors IMC-R117C PIWIL1 Colorectal, gastric, pancreatic IMC-C103C1 MAGE-A4 Multiple solid tumors IMC-I109V Envelope Hepatitis B Virus (HBV) IMC-M113V2 Gag Human Immunodeficiency Virus
(HIV) New Im mTAC candidate 1. Developed under a co-development/co-promotion collaboration with Genentech; 2. Program is wholly owned, development costs being provided by the Bill & Melinda Gates Foundation (BMGF), Immunocore retains all
development and commercialization rights in the developed world. * Platinum refractory or resistant serous ovarian carcinoma