
Transformative Medicines for Patients January 2023

This presentation contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995.
Words such as “may,” “can,” “will,” “believe,” “expect,” “plan,” “anticipate,” “potential” and similar expressions (as well as other words or expressions referencing future events or circumstances) are intended to identify forward-looking
statements. All statements, other than statements of historical facts, included in this presentation are forward-looking statements. These statements include, but are not limited to, statements regarding the marketing, therapeutic potential,
and expected clinical benefits, including extended overall survival benefit and reduction in circulating tumor DNA, of Immunocore’s products and product candidates; expectations regarding the development of Immunocore’s pipeline and the design,
progress, timing, enrollment, scope, expansion and results of Immunocore’s existing, planned and other future clinical trials and IND enabling studies, including the targeted delivery of IND for three new product candidates, the expansion of,
and timing for reporting data from the monotherapy and combination arms of, the PRAME-A02 trial and the timing for reporting data from the single ascending dose portion of the IMC-M113V Phase 1 HIV clinical trial; the ability of TCR
therapeutics to target 90% of the human proteome; statements regarding the durability, efficacy and toleration of Immunocore’s product candidates; expectations regarding the commercialization of KIMMTRAK including potential growth opportunities
and trends and increasing access to KIMMTRAK; expectations regarding the value proposition of KIMMTRAK in metastatic uveal melanoma (mUM) and advanced melanoma; expectations regarding the potential market size and opportunity for Immunocore’s
products and product candidates, including statements with respect to potential patient population; expectations regarding the potential of PRAME-A02 to benefit a large number of patients; the Company’s belief that IMC-R117C is potentially the
first-in-class PIWIL1-targeted immunotherapy for colorectal and other gastrointestinal cancers; statements regarding the planned IND timing for IMC-R117C; expectations regarding future milestones; future development plans of tebentafusp and
Immunocore’s other product candidates; the ability to obtain and maintain regulatory approval for its products and product candidates; expectations regarding the sustained or potential commercial performance and uptake of KIMMTRAK and
Immunocore’s other product candidates, if approved; expectations regarding Immunocore’s management of resources and expected cash runway; and preliminary unaudited net sales and cash and cash equivalents of KIMMTRAK and tebentafusp; and the
validation of the ImmTAC platform. These forward-looking statements are based on management’s current expectations and beliefs and are subject to a number of risks, uncertainties and important factors that may cause actual events or results
to differ materially and adversely from those expressed or implied by any forward-looking statements, many of which are beyond Immunocore’s control. These include, without limitation, risks and uncertainties related to the impact of worsening
macroeconomic conditions and the ongoing and evolving COVID-19 pandemic, the war in Ukraine or global geopolitical tension on Immunocore’s business, strategy, clinical trials, financial position and anticipated milestones, including
Immunocore’s ability to conduct ongoing and planned clinical trials; Immunocore’s ability to obtain and maintain regulatory approval of its product candidates; Immunocore’s ability to obtain clinical supply of current or future product
candidates or commercial supply of KIMMTRAK or any future approved products, including as a result of supply chain disruptions; Immunocore’s ability to develop, manufacture and commercialize its product candidates; Immunocore’s ability and
plans to launch, market and sell KIMMTRAK or any future approved products, to continue to establish and expand a commercial infrastructure; Immunocore’s ability to successfully expand the approved indications for KIMMTRAK, or obtain marketing
approval for KIMMTRAK in additional geographies in the future; the delay of any current or planned clinical trials, whether due to the COVID-19 pandemic, patient enrollment delays or otherwise; unexpected safety or efficacy data observed during
preclinical studies or clinical trials and Immunocore’s ability to successfully demonstrate the safety and efficacy of its product candidates and gain approval of its product candidates on a timely basis, if at all; competition with respect to
market opportunities; actions of regulatory agencies, which may affect the initiation, timing and progress of clinical trials or future regulatory approval; Immunocore’s ability to obtain, maintain and enforce intellectual property protection
for KIMMTRAK or any product candidates it is developing; clinical trial site activation or enrollment rates that are lower than expected; Immunocore’s need for and ability to obtain additional funding on favorable terms or at all, including as
a result of worsening macroeconomic conditions such as rising inflation and interest rates, volatility in the capital markets and related market uncertainty; and the success of Immunocore’s current and future collaborations, partnerships or
licensing arrangements. These and other risks and uncertainties are described in greater detail in the section titled ‘Risk Factors’; in Immunocore’s filings with the Securities and Exchange Commission, including Immunocore’s most recent Annual
Report on Form 20-F, as supplemented by its most recent filings that Immunocore has made or may make with the SEC in the future. Such risks may be amplified by the COVID-19 pandemic and its potential impact on Immunocore’s business and the
overall global economy. Any forward-looking statements represent Immunocore’s views only as of the date of this presentation and should not be relied upon as representing its views as of any subsequent date. Immunocore does not assume any
obligation to update any forward-looking statements, except as may be required by law. In addition, as the reported net sales and cash and cash equivalents in this presentation are preliminary, have not been audited and are subject to change
pending completion of our audited financial statements for the year ended December 31, 2022, it is possible that Immunocore or its independent registered public accounting firm may identify items that require Immunocore to make adjustments to
the amount included in this presentation, and such changes could be material. Additional information and disclosures would also be required for a more complete understanding of Immunocore’s financial position and results of operations as of
December 31, 2022. Certain information contained in this presentation relates to or is based on studies, publications, surveys, and other data obtained from third-party sources and Immunocore’s own internal estimates and research. While
Immunocore believes these third-party sources to be reliable as of the date of this presentation, it has not independently verified, and makes no representation as to the adequacy, fairness, accuracy, or completeness of, any information
obtained from third-party sources. KIMMTRAK™ is a trademark owned or licensed to Immunocore. Forward-looking statement 2
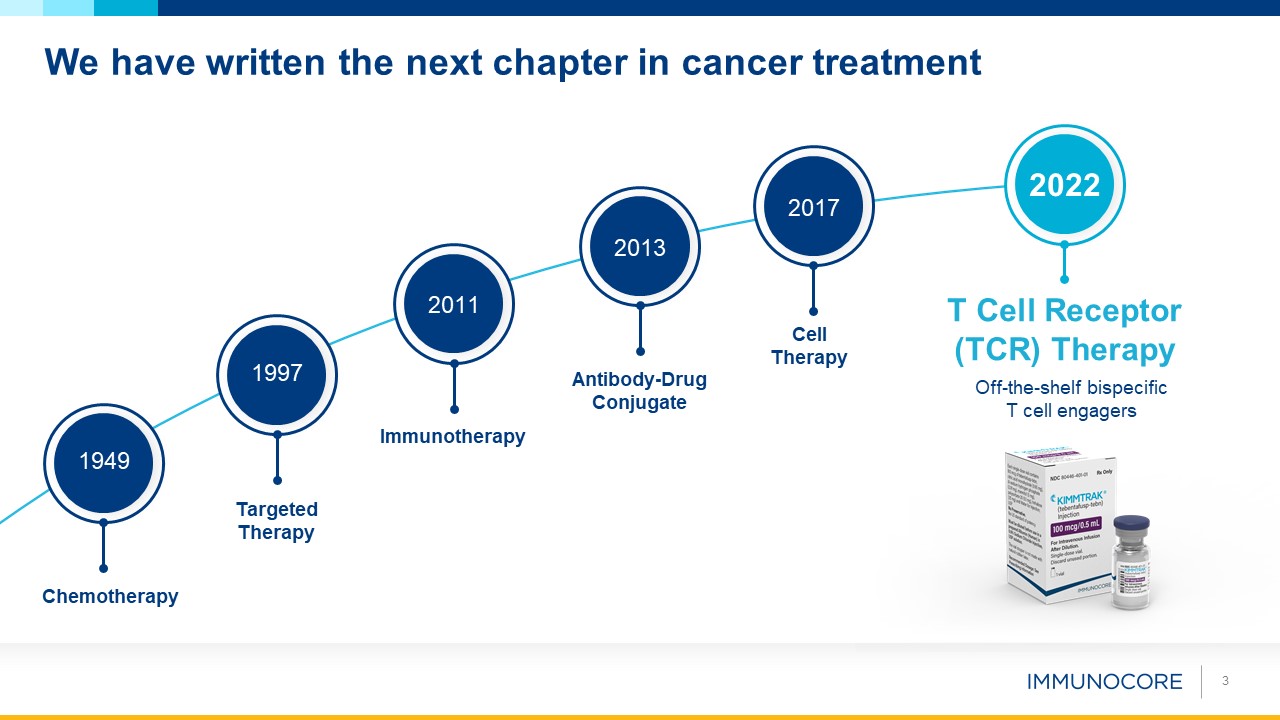
We have written the next chapter in cancer treatment 3 T Cell Receptor (TCR) Therapy Off-the-shelf bispecific T cell
engagers Chemotherapy 1949 Targeted Therapy 1997 Immunotherapy 2011 Antibody-DrugConjugate 2013 Cell Therapy 2017 2022
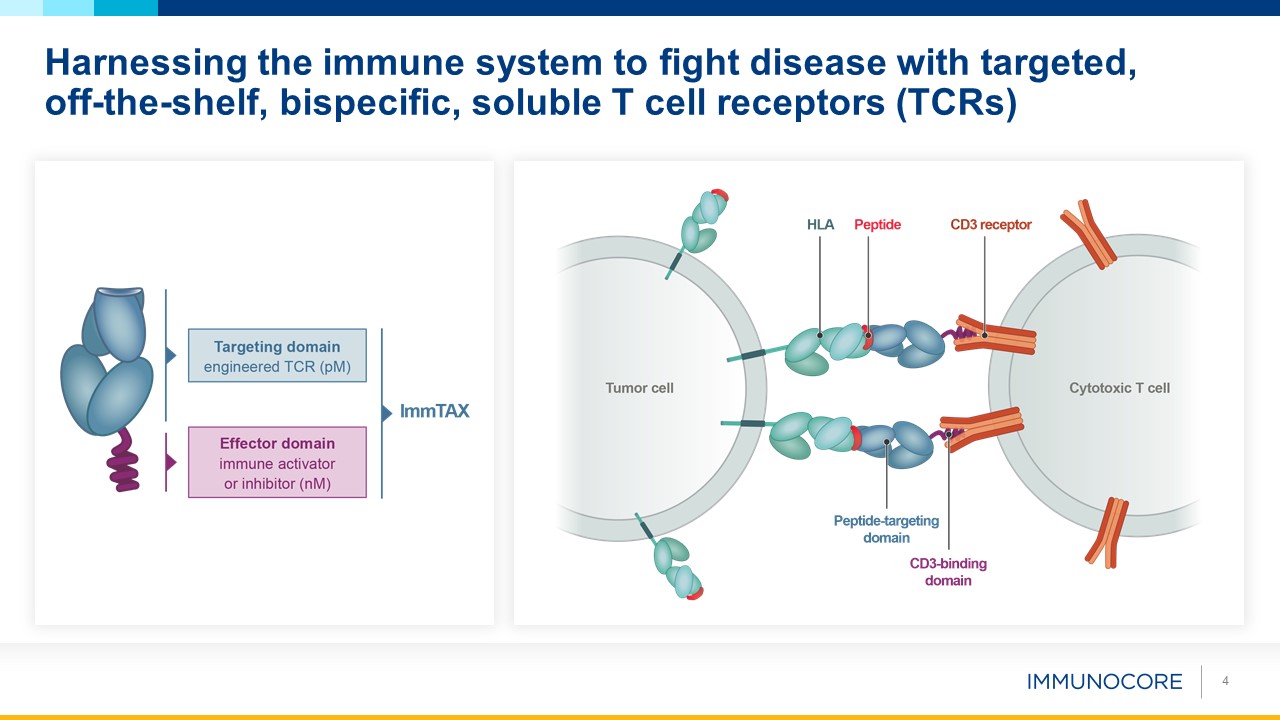
4 Harnessing the immune system to fight disease with targeted, off-the-shelf, bispecific, soluble T cell receptors (TCRs)
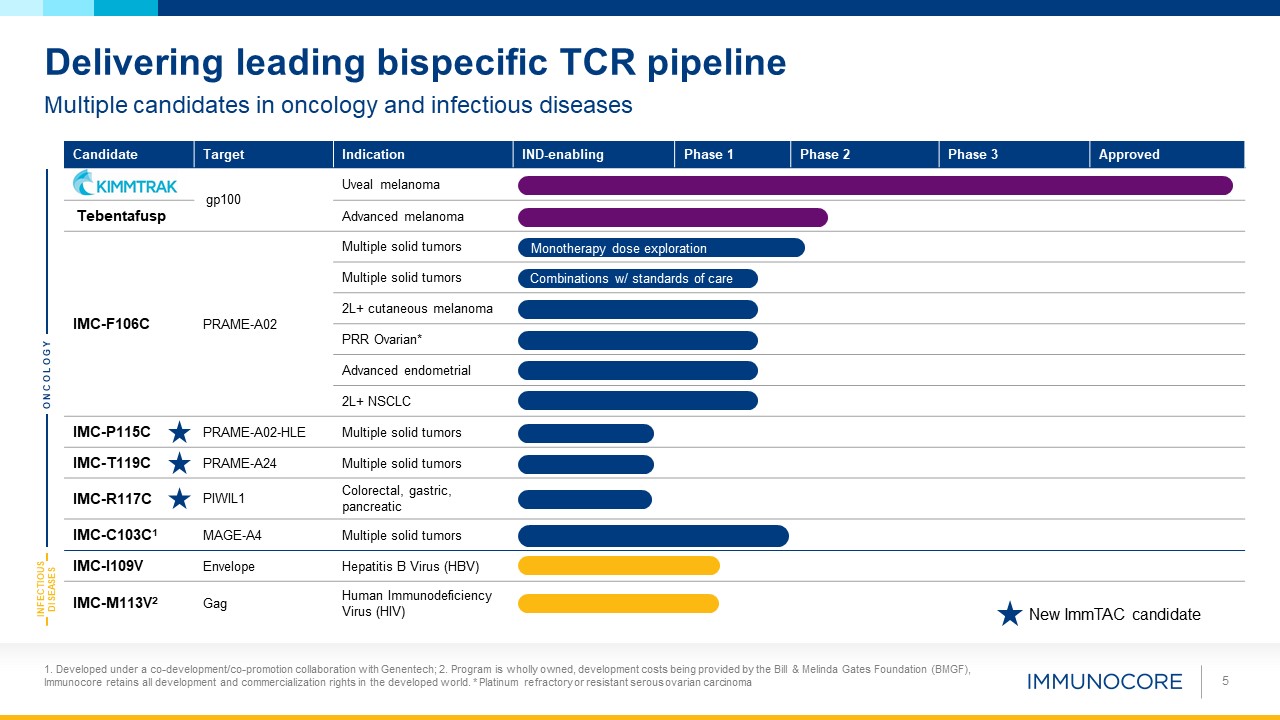
5 Multiple candidates in oncology and infectious diseases Delivering leading bispecific TCR
pipeline Candidate Target Indication IND-enabling Phase 1 Phase 2 Phase 3 Approved gp100 Uveal melanoma Tebentafusp Advanced melanoma IMC-F106C PRAME-A02 Multiple solid tumors Multiple solid tumors 2L+ cutaneous melanoma PRR
Ovarian* Advanced endometrial 2L+ NSCLC IMC-P115C PRAME-A02-HLE Multiple solid tumors IMC-T119C PRAME-A24 Multiple solid tumors IMC-R117C PIWIL1 Colorectal, gastric, pancreatic IMC-C103C1 MAGE-A4 Multiple solid
tumors IMC-I109V Envelope Hepatitis B Virus (HBV) IMC-M113V2 Gag Human Immunodeficiency Virus (HIV) ONCOLOGY INFECTIOUS DISEASES Monotherapy dose exploration Combinations w/ standards of care New ImmTAC candidate 1. Developed under
a co-development/co-promotion collaboration with Genentech; 2. Program is wholly owned, development costs being provided by the Bill & Melinda Gates Foundation (BMGF), Immunocore retains all development and commercialization rights in the
developed world. * Platinum refractory or resistant serous ovarian carcinoma

Technology platform
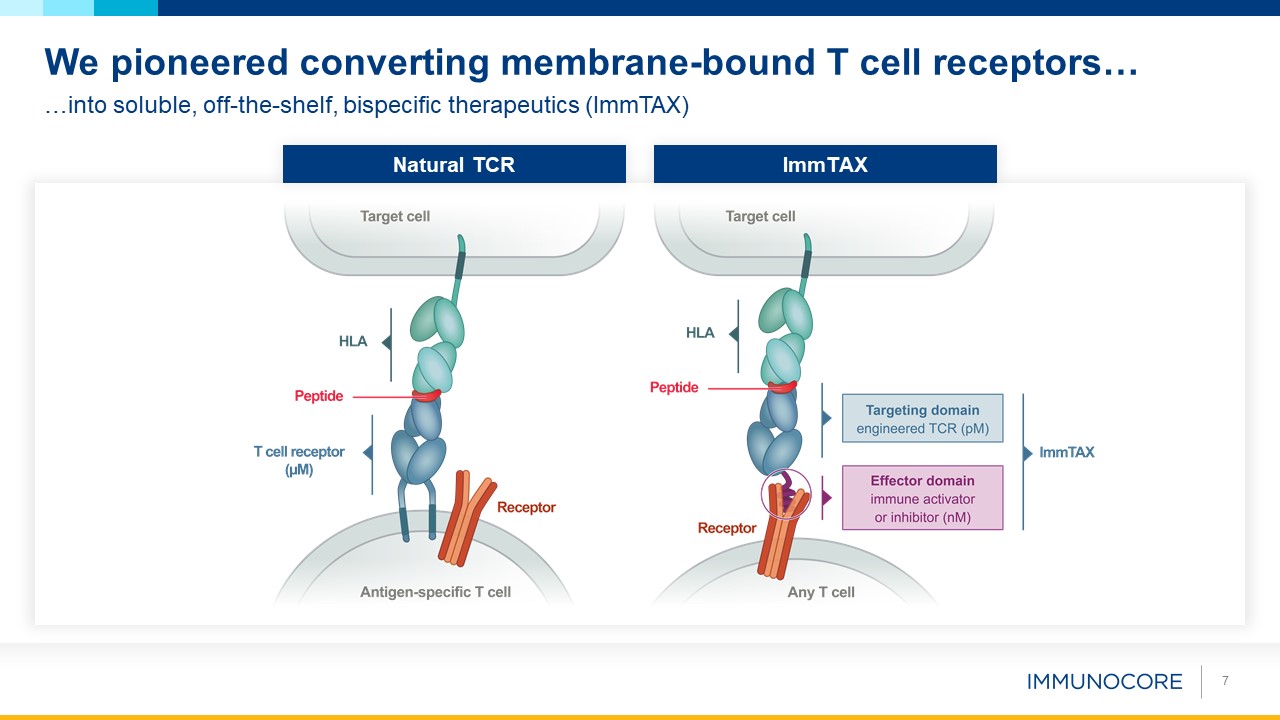
7 …into soluble, off-the-shelf, bispecific therapeutics (ImmTAX) We pioneered converting membrane-bound T cell receptors… Natural
TCR ImmTAX
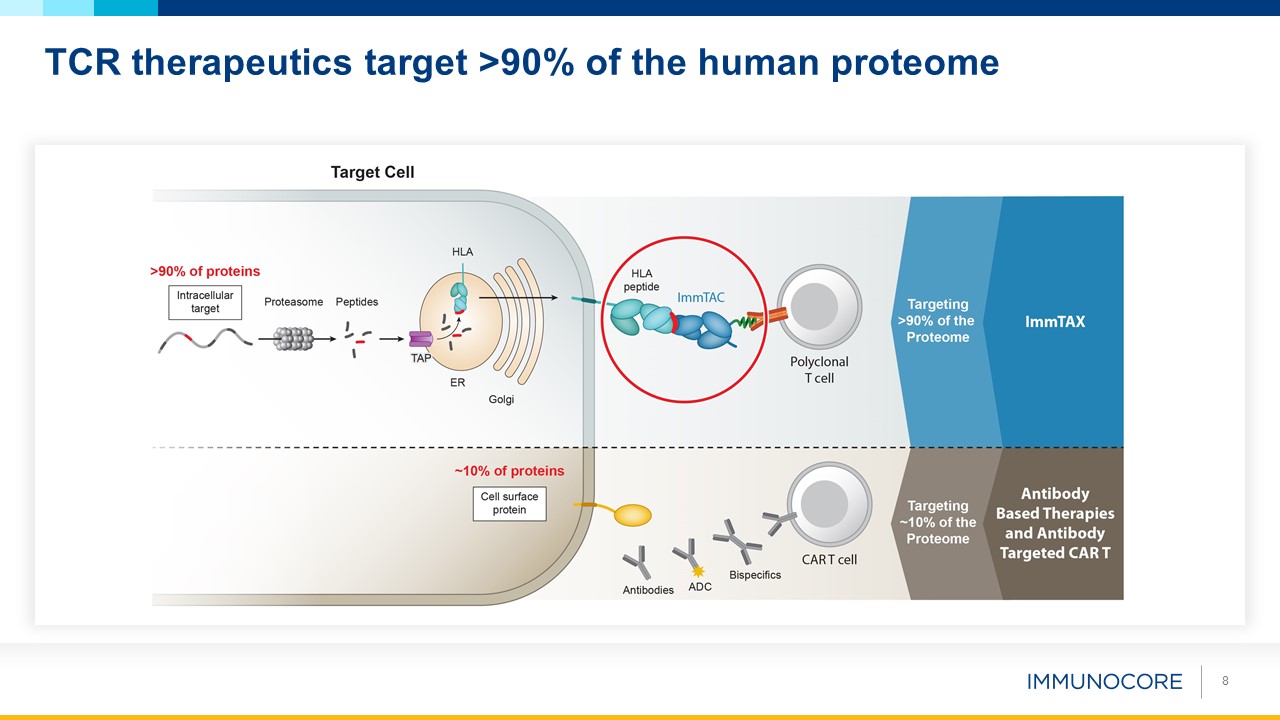
8 TCR therapeutics target >90% of the human proteome
Our platform is modular Applicable across 3 therapeutic areas 9 Oncology Infectious Diseases Autoimmune
Conditions UPREGULATION OF THE IMMUNE SYSTEM DOWNREGULATION OF THE IMMUNE SYSTEM

KIMMTRAK® in metastatic melanoma

11 We are using our TCR technology to target gp100 protein in melanoma Metastatic Uveal Melanoma (mUM): is an ultra-rare and
aggressive tumor ~1,000 Until KIMMTRAK, no approved treatment3 Originates from melanocytes within the uveal tract of the eye Median age at diagnosis is 62 years1 HLA-02 mUM pts per year in the US/EU2 Up to 50% may develop metastatic
disease; liver primary site of metastasis1 Historic median survival with metastatic disease2 ~12 months 1. Yang J et al. Ther Adv Med Oncol. 2018 ; 2. Carvajal RD et al. Br J Ophthalmol. 2017; 3. Rantala ES et al. Melanoma Res. Published
online. 2019
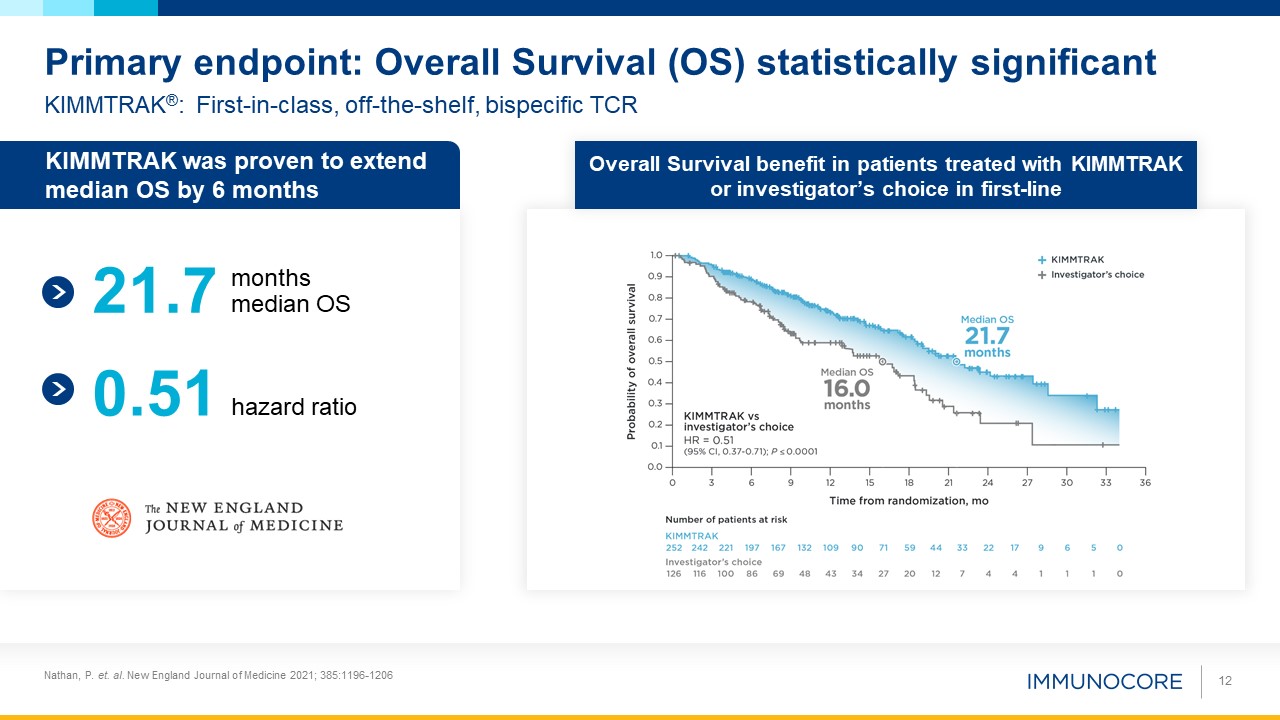
Nathan, P. et. al. New England Journal of Medicine 2021; 385:1196-1206 12 KIMMTRAK®: First-in-class, off-the-shelf, bispecific
TCR Primary endpoint: Overall Survival (OS) statistically significant Overall Survival benefit in patients treated with KIMMTRAK or investigator’s choice in first-line KIMMTRAK was proven to extend median OS by 6 months months median
OS hazard ratio 21.7 0.51
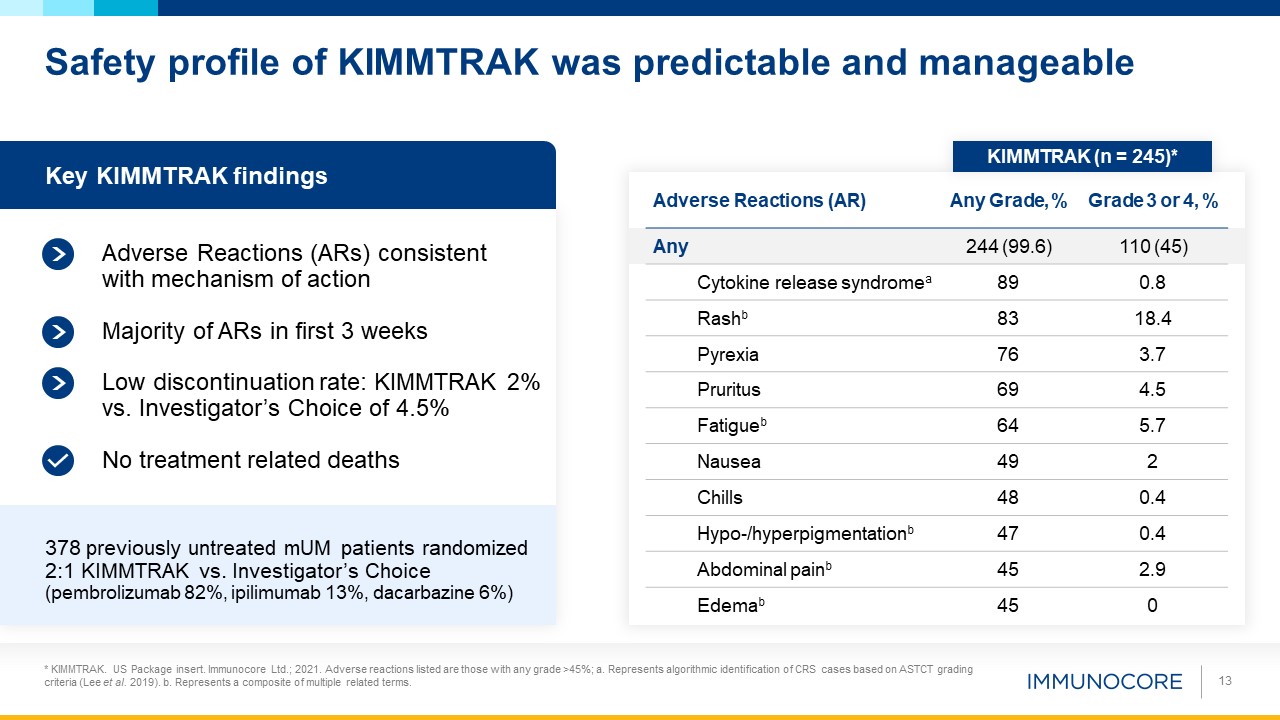
Safety profile of KIMMTRAK was predictable and manageable 13 Key KIMMTRAK findings No treatment related deaths 378 previously
untreated mUM patients randomized 2:1 KIMMTRAK vs. Investigator’s Choice (pembrolizumab 82%, ipilimumab 13%, dacarbazine 6%) Majority of ARs in first 3 weeks Low discontinuation rate: KIMMTRAK 2% vs. Investigator’s Choice of 4.5% Adverse
Reactions (AR) Any Grade, % Grade 3 or 4, % Any 244 (99.6) 110 (45) Cytokine release
syndromea 89 0.8 Rashb 83 18.4 Pyrexia 76 3.7 Pruritus 69 4.5 Fatigueb 64 5.7 Nausea 49 2 Chills 48 0.4 Hypo-/hyperpigmentationb 47 0.4 Abdominal painb 45 2.9 Edemab 45 0 Adverse Reactions (ARs) consistent with
mechanism of action KIMMTRAK (n = 245)* * KIMMTRAK. US Package insert. Immunocore Ltd.; 2021. Adverse reactions listed are those with any grade >45%; a. Represents algorithmic identification of CRS cases based on ASTCT grading criteria
(Lee et al. 2019). b. Represents a composite of multiple related terms.
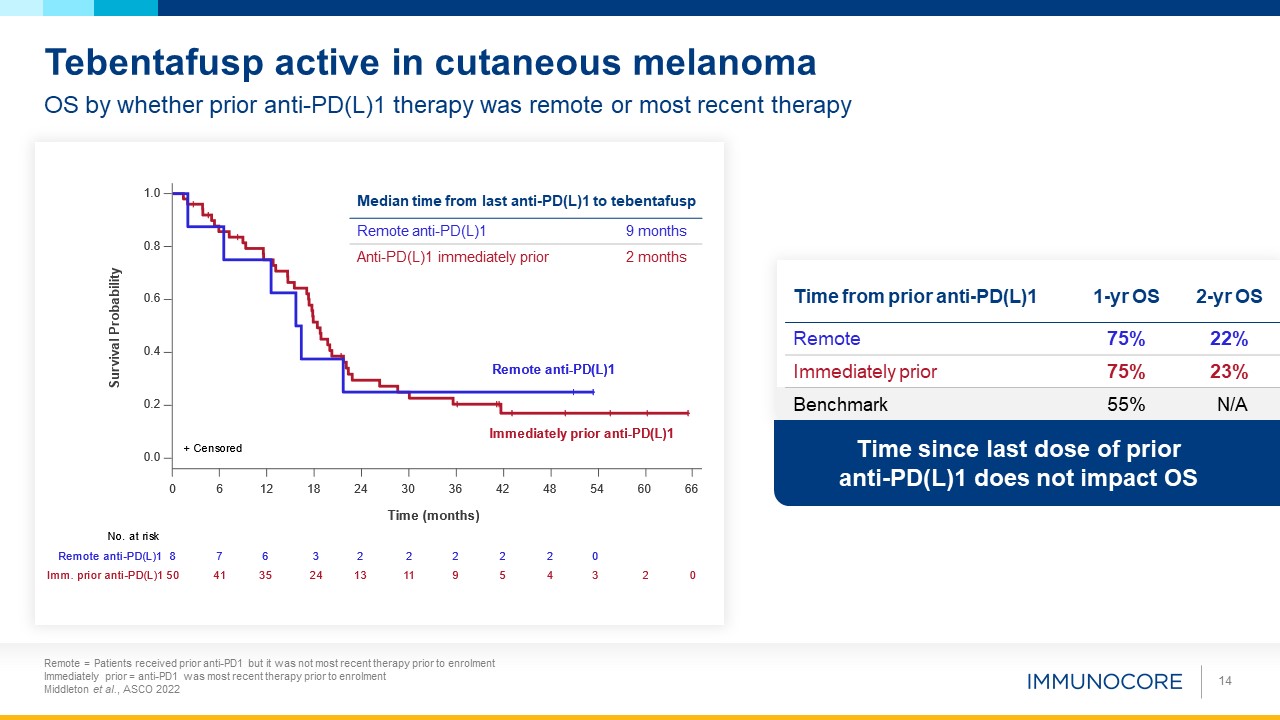
Time since last dose of prior anti-PD(L)1 does not impact OS 14 OS by whether prior anti-PD(L)1 therapy was remote or most recent
therapy Tebentafusp active in cutaneous melanoma Time from prior anti-PD(L)1 1-yr OS 2-yr OS Remote 75% 22% Immediately prior 75% 23% Benchmark 55% N/A Median time from last anti-PD(L)1 to tebentafusp 2-yr OS Remote
anti-PD(L)1 9 months Anti-PD(L)1 immediately prior 2 months Remote = Patients received prior anti-PD1 but it was not most recent therapy prior to enrolment Immediately prior = anti-PD1 was most recent therapy prior to enrolment Middleton
et al., ASCO 2022
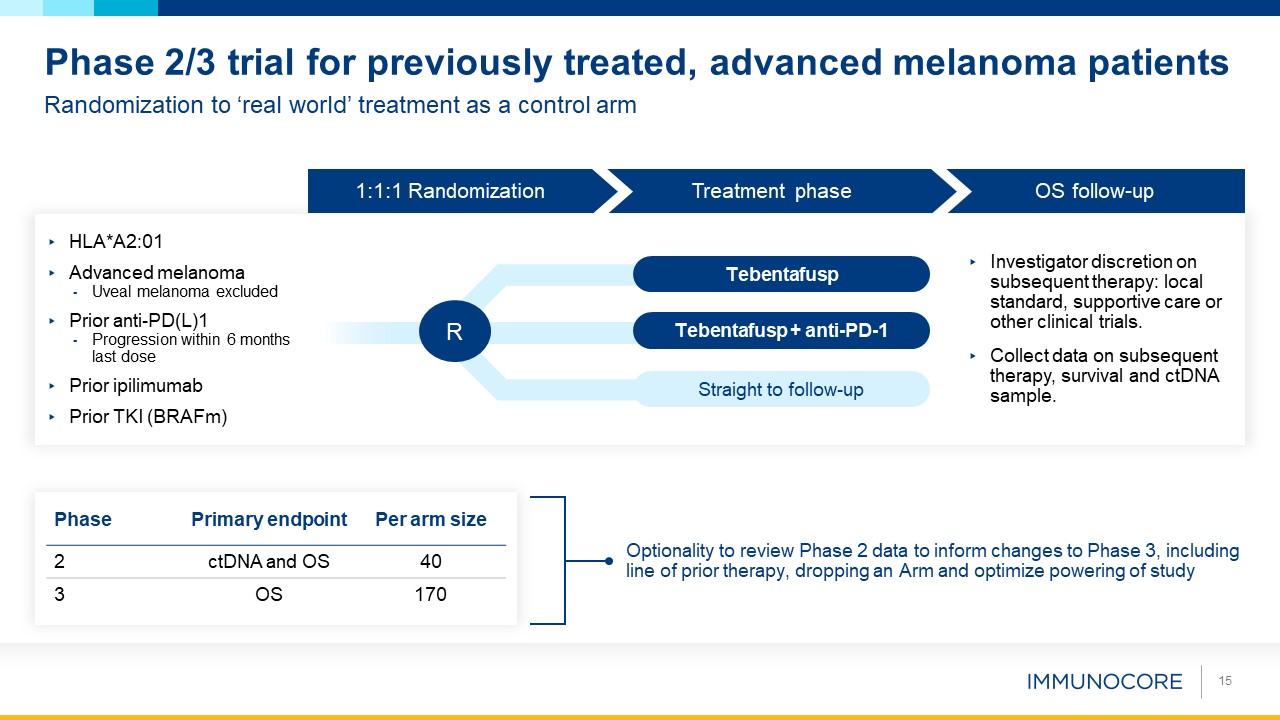
15 Randomization to ‘real world’ treatment as a control arm Phase 2/3 trial for previously treated, advanced melanoma
patients Phase Primary endpoint Per arm size 2 ctDNA and OS 40 3 OS 170 Optionality to review Phase 2 data to inform changes to Phase 3, including line of prior therapy, dropping an Arm and optimize powering of study Investigator
discretion on subsequent therapy: local standard, supportive care or other clinical trials. Collect data on subsequent therapy, survival and ctDNA sample. HLA*A2:01 Advanced melanoma Uveal melanoma excluded Prior anti-PD(L)1 Progression
within 6 months last dose Prior ipilimumab Prior TKI (BRAFm) 1:1:1 Randomization Treatment phase OS follow-up Tebentafusp Tebentafusp + anti-PD-1 R Straight to follow-up

PRAME Franchise:A02, A24, A02-HLE
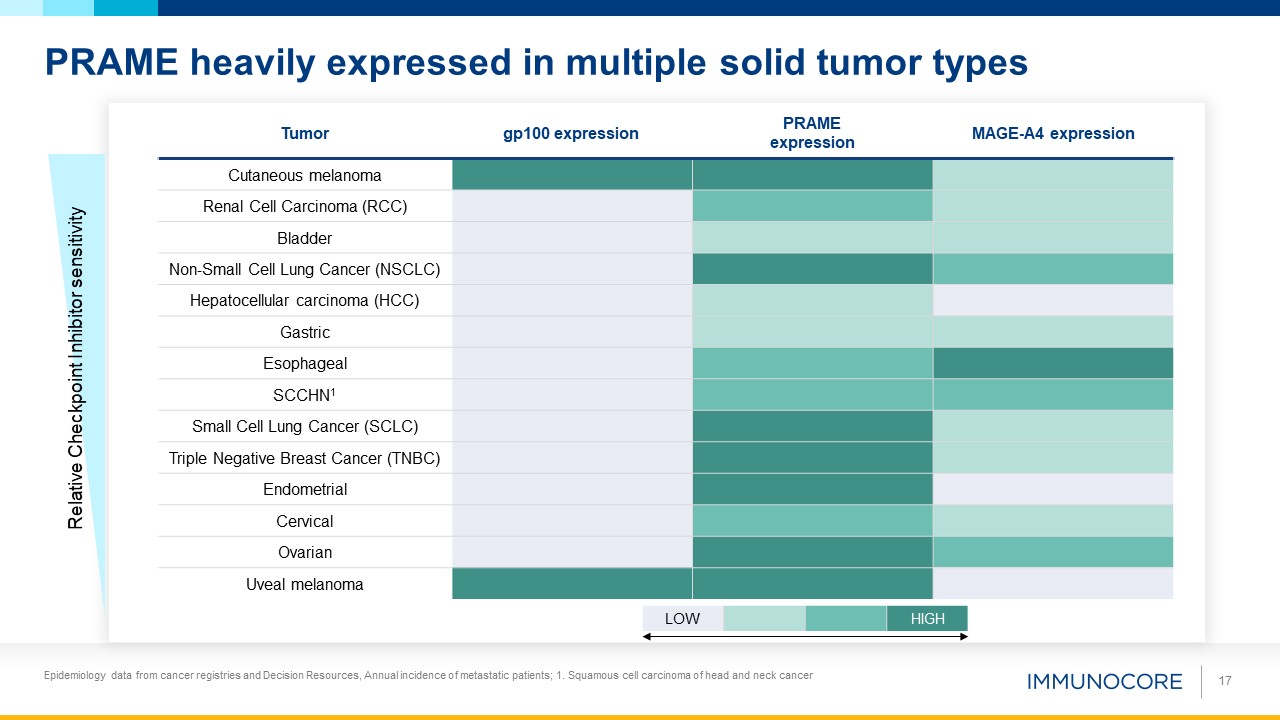
PRAME heavily expressed in multiple solid tumor types LOW HIGH Tumor gp100 expression PRAME expression MAGE-A4
expression Cutaneous melanoma Renal Cell Carcinoma (RCC) Bladder Non-Small Cell Lung Cancer (NSCLC) Hepatocellular carcinoma (HCC) Gastric Esophageal SCCHN1 Small Cell Lung Cancer (SCLC) Triple Negative Breast Cancer
(TNBC) Endometrial Cervical Ovarian Uveal melanoma Relative Checkpoint Inhibitor sensitivity 17 Epidemiology data from cancer registries and Decision Resources, Annual incidence of metastatic patients; 1. Squamous cell carcinoma of head
and neck cancer
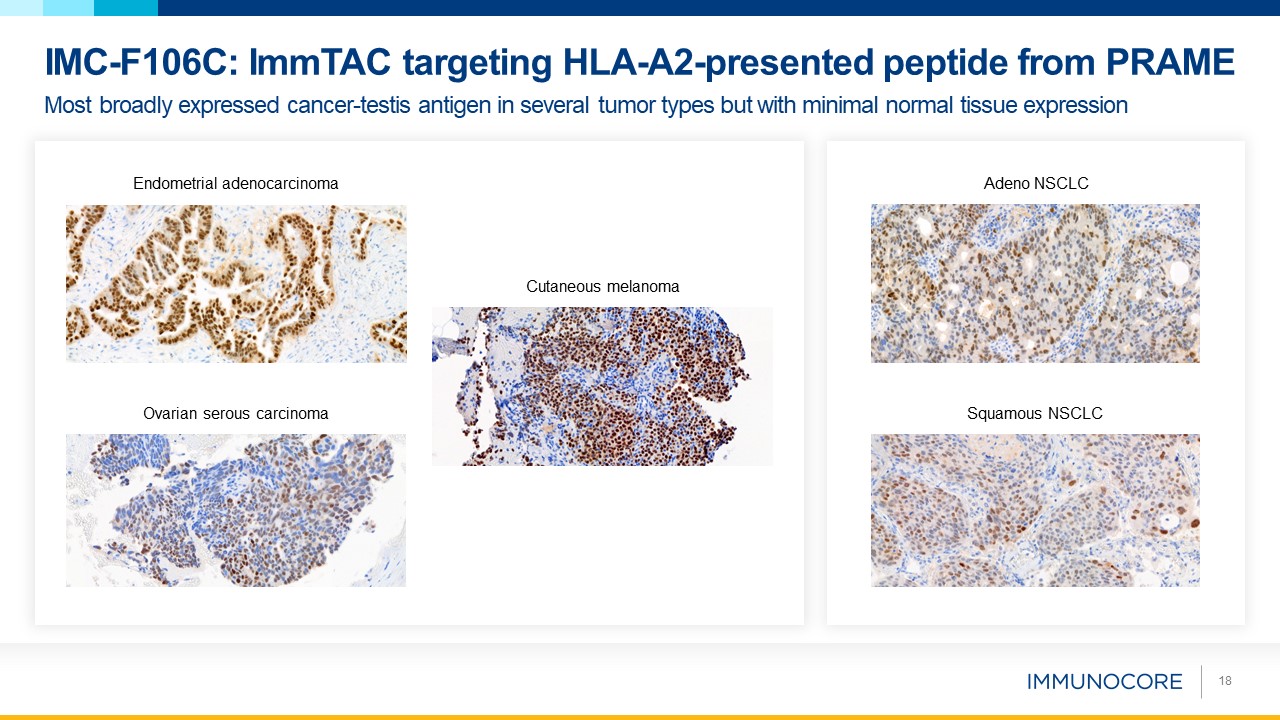
18 Most broadly expressed cancer-testis antigen in several tumor types but with minimal normal tissue expression IMC-F106C: ImmTAC
targeting HLA-A2-presented peptide from PRAME Squamous NSCLC Adeno NSCLC Ovarian serous carcinoma Cutaneous melanoma Endometrial adenocarcinoma
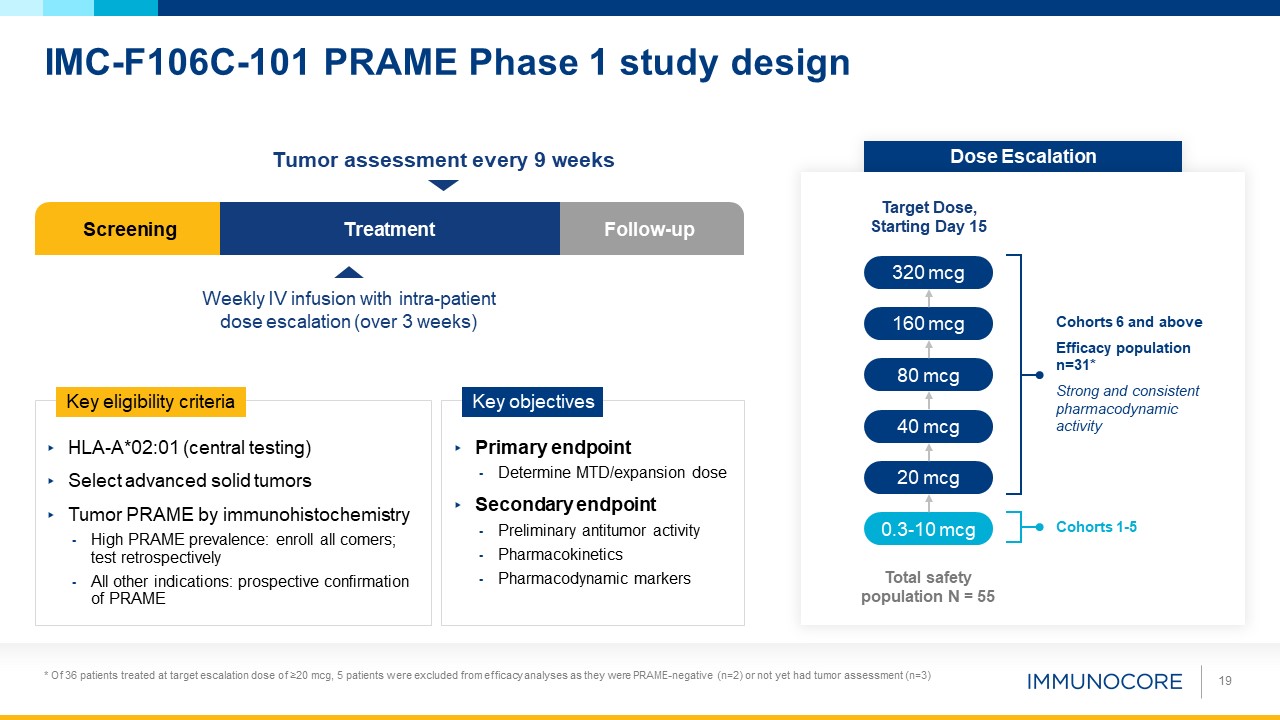
IMC-F106C-101 PRAME Phase 1 study design 19 Screening Treatment Follow-up Weekly IV infusion with intra-patient dose escalation
(over 3 weeks) Tumor assessment every 9 weeks HLA-A*02:01 (central testing) Select advanced solid tumors Tumor PRAME by immunohistochemistry High PRAME prevalence: enroll all comers; test retrospectively All other indications: prospective
confirmation of PRAME Key eligibility criteria Primary endpoint Determine MTD/expansion dose Secondary endpoint Preliminary antitumor activity Pharmacokinetics Pharmacodynamic markers Key objectives Dose Escalation Target Dose,
Starting Day 15 0.3-10 mcg 20 mcg 40 mcg 80 mcg 160 mcg 320 mcg Total safety population N = 55 Cohorts 1-5 Cohorts 6 and above Efficacy population n=31* Strong and consistent pharmacodynamic activity * Of 36 patients treated at
target escalation dose of ≥20 mcg, 5 patients were excluded from efficacy analyses as they were PRAME-negative (n=2) or not yet had tumor assessment (n=3)
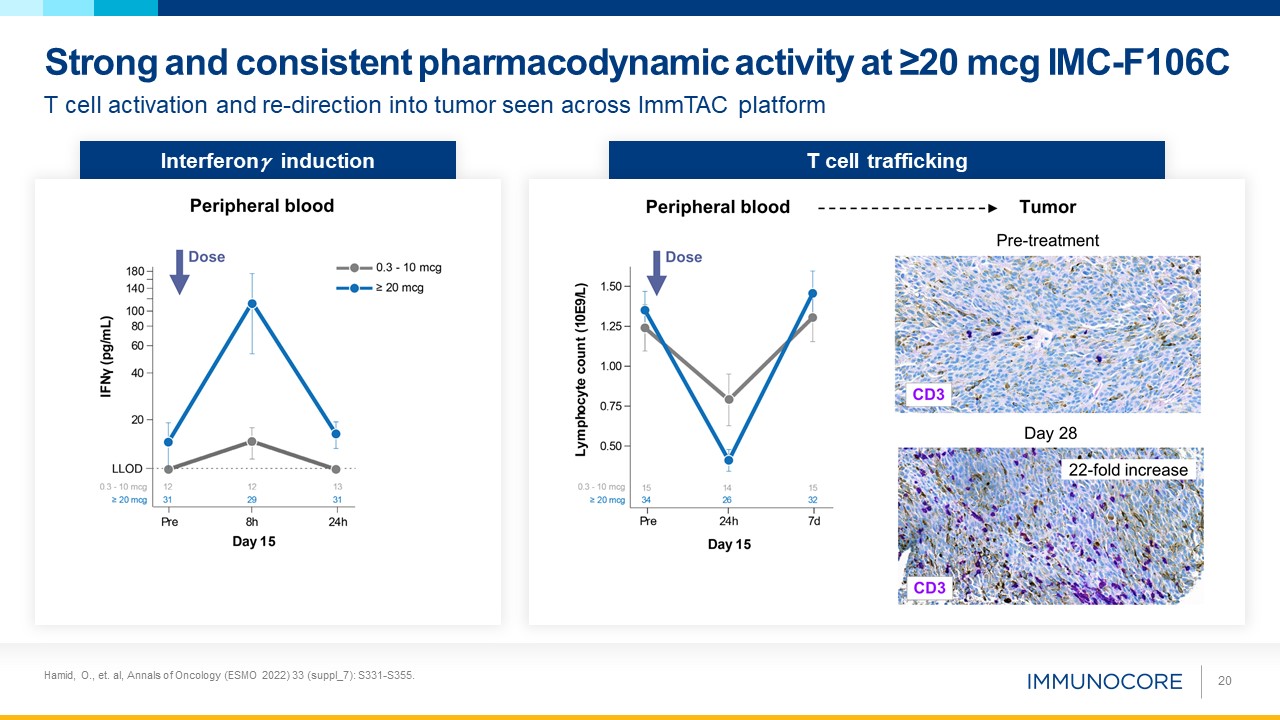
20 T cell activation and re-direction into tumor seen across ImmTAC platform Strong and consistent pharmacodynamic activity at ≥20
mcg IMC-F106C Interferon induction T cell trafficking Hamid, O., et. al, Annals of Oncology (ESMO 2022) 33 (suppl_7): S331-S355.
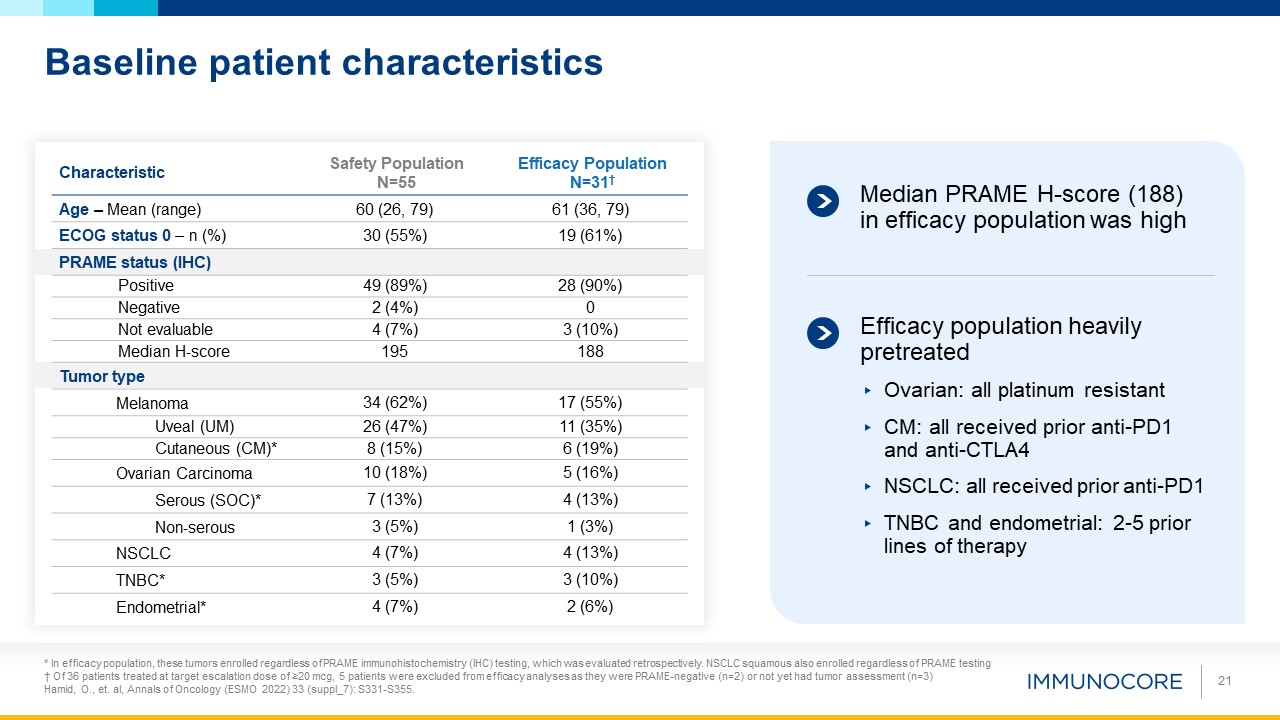
Baseline patient characteristics 21 Characteristic Safety Population N=55 Efficacy Population N=31† Age – Mean (range) 60
(26, 79) 61 (36, 79) ECOG status 0 – n (%) 30 (55%) 19 (61%) PRAME status (IHC) Positive 49 (89%) 28 (90%) Negative 2 (4%) 0 Not evaluable 4 (7%) 3 (10%) Median H-score 195 188 Tumor type Melanoma 34 (62%) 17
(55%) Uveal (UM) 26 (47%) 11 (35%) Cutaneous (CM)* 8 (15%) 6 (19%) Ovarian Carcinoma 10 (18%) 5 (16%) Serous (SOC)* 7 (13%) 4 (13%) Non-serous 3 (5%) 1 (3%) NSCLC 4 (7%) 4 (13%) TNBC* 3 (5%) 3 (10%) Endometrial* 4
(7%) 2 (6%) Median PRAME H-score (188) in efficacy population was high Efficacy population heavily pretreated Ovarian: all platinum resistant CM: all received prior anti-PD1 and anti-CTLA4 NSCLC: all received prior anti-PD1 TNBC and
endometrial: 2-5 prior lines of therapy * In efficacy population, these tumors enrolled regardless of PRAME immunohistochemistry (IHC) testing, which was evaluated retrospectively. NSCLC squamous also enrolled regardless of PRAME testing †
Of 36 patients treated at target escalation dose of ≥20 mcg, 5 patients were excluded from efficacy analyses as they were PRAME-negative (n=2) or not yet had tumor assessment (n=3) Hamid, O., et. al, Annals of Oncology (ESMO 2022) 33
(suppl_7): S331-S355.
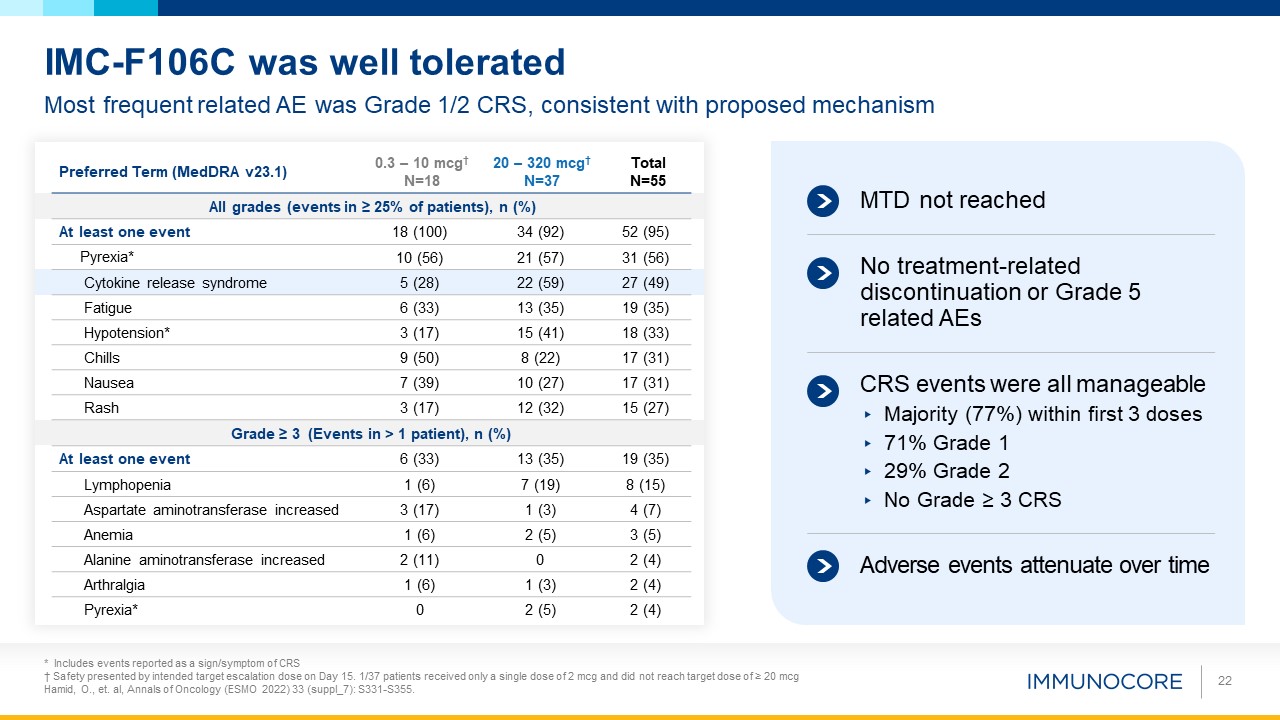
Most frequent related AE was Grade 1/2 CRS, consistent with proposed mechanism IMC-F106C was well tolerated MTD not reached CRS
events were all manageable Majority (77%) within first 3 doses 71% Grade 1 29% Grade 2 No Grade ≥ 3 CRS No treatment-related discontinuation or Grade 5 related AEs Adverse events attenuate over time Preferred Term (MedDRA v23.1) 0.3
– 10 mcg†N=18 20 – 320 mcg†N=37 Total N=55 All grades (events in ≥ 25% of patients), n (%) At least one event 18 (100) 34 (92) 52 (95) Pyrexia* 10 (56) 21 (57) 31 (56) Cytokine release syndrome 5 (28) 22 (59) 27 (49) Fatigue 6
(33) 13 (35) 19 (35) Hypotension* 3 (17) 15 (41) 18 (33) Chills 9 (50) 8 (22) 17 (31) Nausea 7 (39) 10 (27) 17 (31) Rash 3 (17) 12 (32) 15 (27) Grade ≥ 3 (Events in > 1 patient), n (%) At least one event 6 (33) 13
(35) 19 (35) Lymphopenia 1 (6) 7 (19) 8 (15) Aspartate aminotransferase increased 3 (17) 1 (3) 4 (7) Anemia 1 (6) 2 (5) 3 (5) Alanine aminotransferase increased 2 (11) 0 2 (4) Arthralgia 1 (6) 1 (3) 2 (4) Pyrexia* 0 2
(5) 2 (4) 22 * Includes events reported as a sign/symptom of CRS † Safety presented by intended target escalation dose on Day 15. 1/37 patients received only a single dose of 2 mcg and did not reach target dose of ≥ 20 mcg Hamid, O., et.
al, Annals of Oncology (ESMO 2022) 33 (suppl_7): S331-S355.
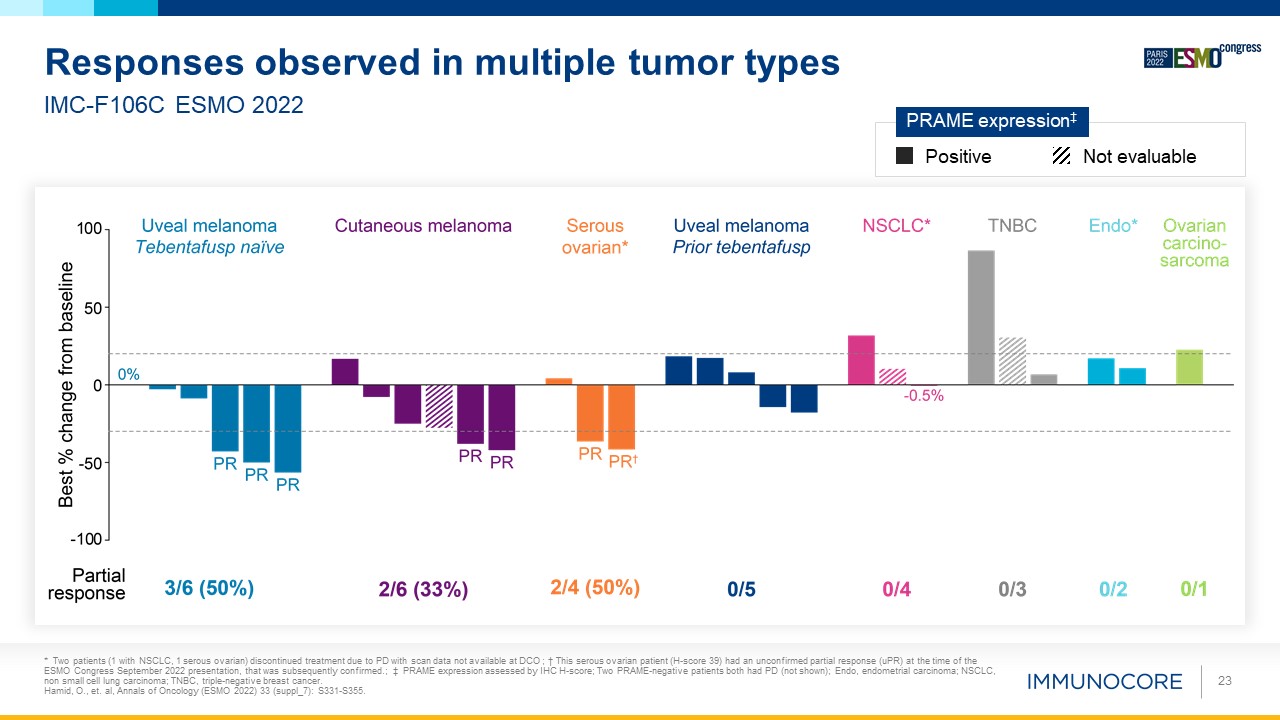
* Two patients (1 with NSCLC, 1 serous ovarian) discontinued treatment due to PD with scan data not available at DCO ; † This serous
ovarian patient (H-score 39) had an unconfirmed partial response (uPR) at the time of the ESMO Congress September 2022 presentation, that was subsequently confirmed.; ‡ PRAME expression assessed by IHC H-score; Two PRAME-negative patients both
had PD (not shown); Endo, endometrial carcinoma; NSCLC, non small cell lung carcinoma; TNBC, triple-negative breast cancer. Hamid, O., et. al, Annals of Oncology (ESMO 2022) 33 (suppl_7): S331-S355. 23 IMC-F106C ESMO 2022 Responses observed
in multiple tumor types Positive Not evaluable PRAME expression‡
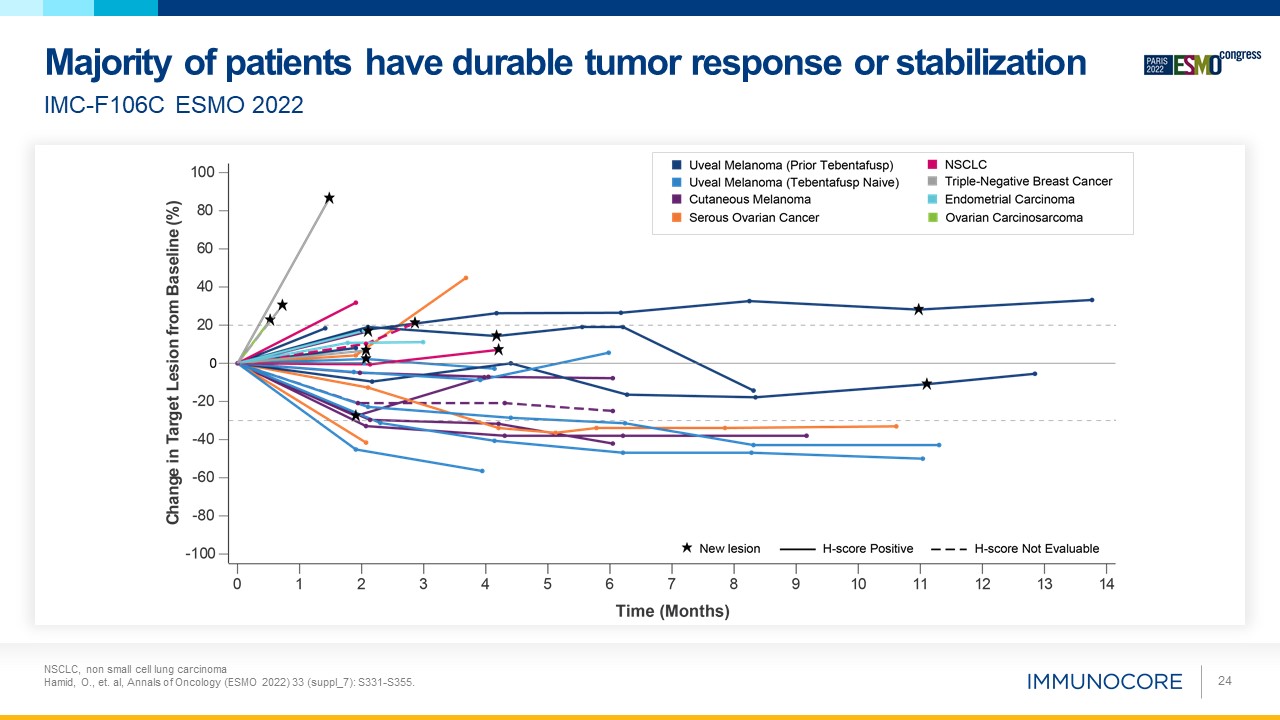
NSCLC, non small cell lung carcinoma Hamid, O., et. al, Annals of Oncology (ESMO 2022) 33 (suppl_7): S331-S355. 24 IMC-F106C ESMO
2022 Majority of patients have durable tumor response or stabilization
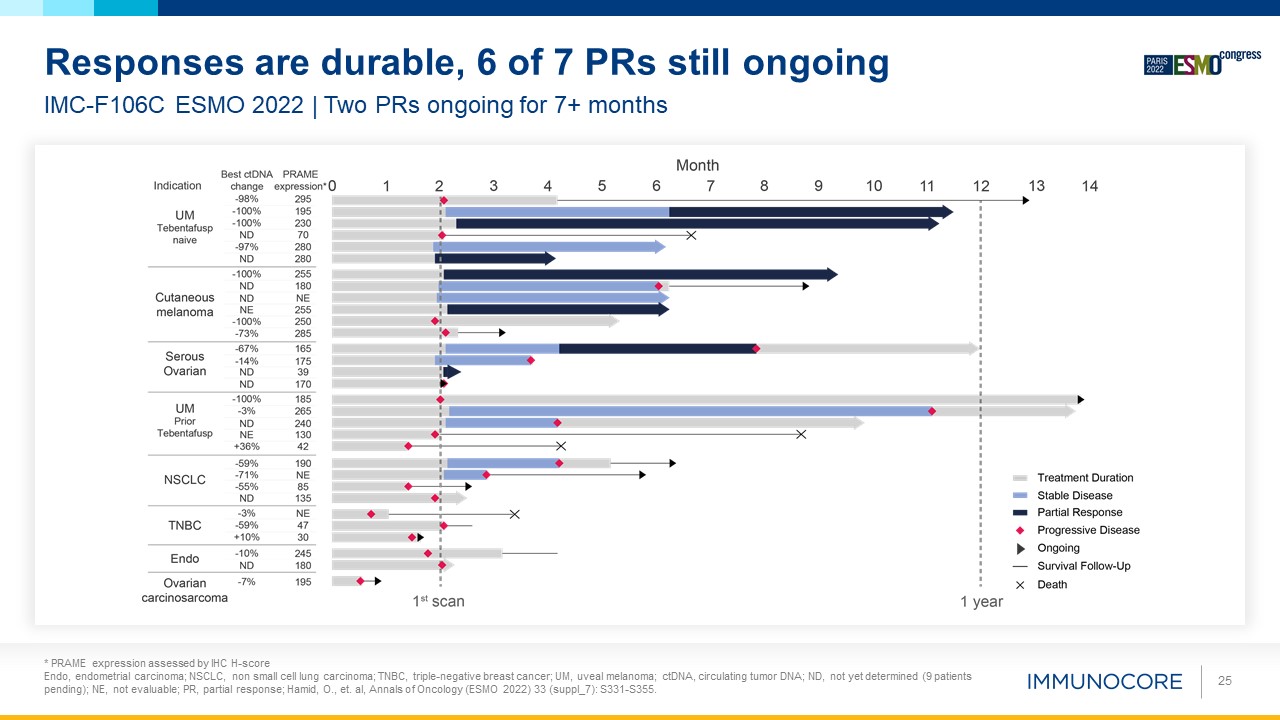
25 IMC-F106C ESMO 2022 | Two PRs ongoing for 7+ months Responses are durable, 6 of 7 PRs still ongoing * PRAME expression
assessed by IHC H-score Endo, endometrial carcinoma; NSCLC, non small cell lung carcinoma; TNBC, triple-negative breast cancer; UM, uveal melanoma; ctDNA, circulating tumor DNA; ND, not yet determined (9 patients pending); NE, not evaluable;
PR, partial response; Hamid, O., et. al, Annals of Oncology (ESMO 2022) 33 (suppl_7): S331-S355.
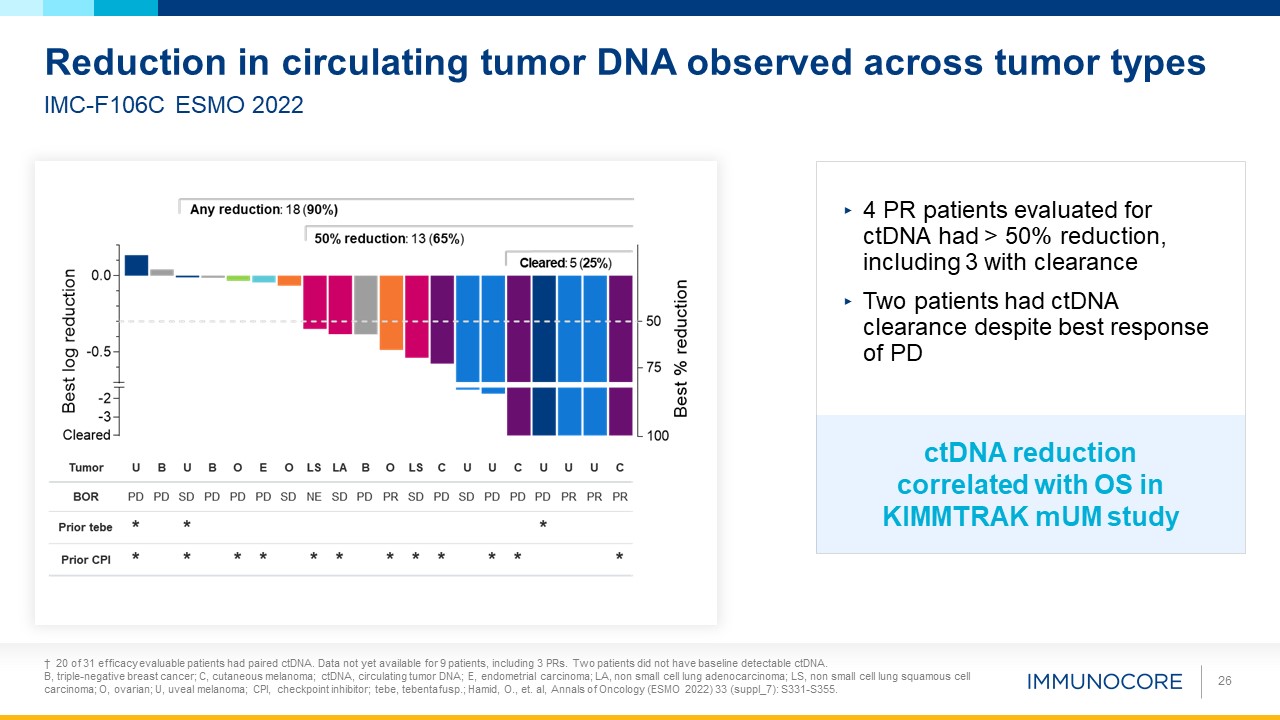
† 20 of 31 efficacy evaluable patients had paired ctDNA. Data not yet available for 9 patients, including 3 PRs. Two patients did
not have baseline detectable ctDNA. B, triple-negative breast cancer; C, cutaneous melanoma; ctDNA, circulating tumor DNA; E, endometrial carcinoma; LA, non small cell lung adenocarcinoma; LS, non small cell lung squamous cell carcinoma; O,
ovarian; U, uveal melanoma; CPI, checkpoint inhibitor; tebe, tebentafusp.; Hamid, O., et. al, Annals of Oncology (ESMO 2022) 33 (suppl_7): S331-S355. 26 IMC-F106C ESMO 2022 Reduction in circulating tumor DNA observed across tumor types 4 PR
patients evaluated for ctDNA had > 50% reduction, including 3 with clearance Two patients had ctDNA clearance despite best response of PD ctDNA reduction correlated with OS in KIMMTRAK mUM study
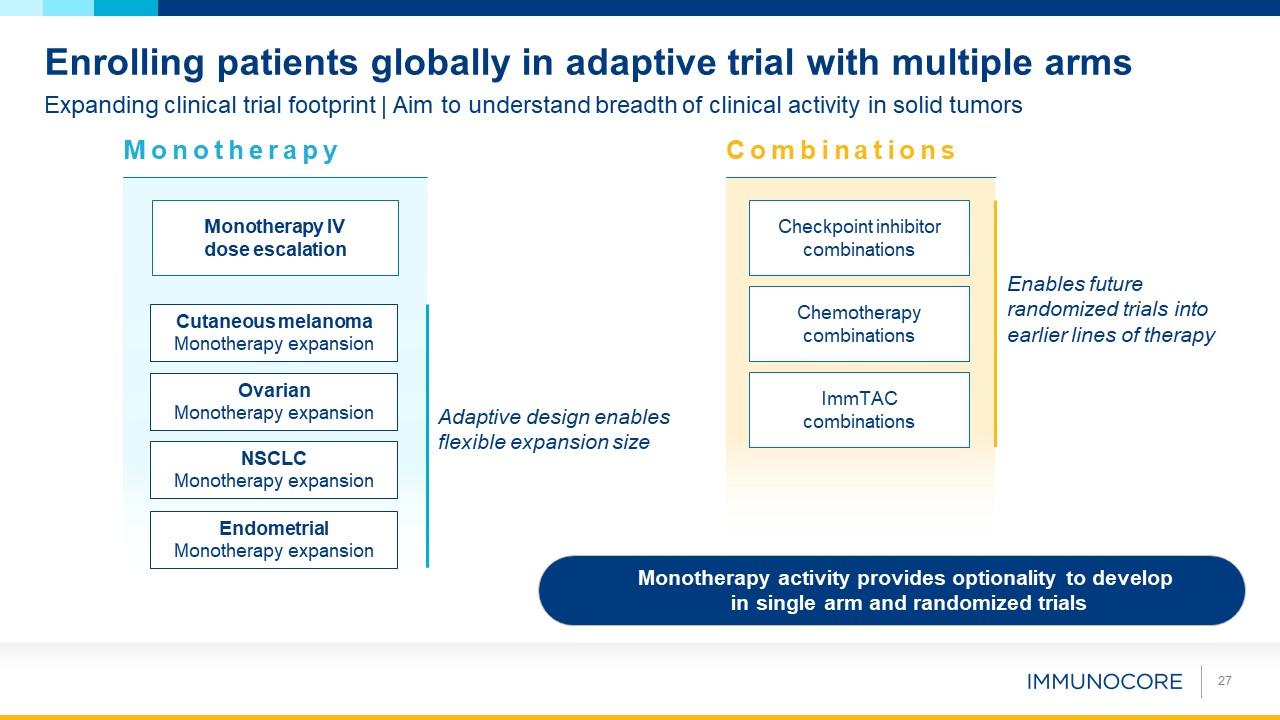
Monotherapy activity provides optionality to develop in single arm and randomized trials 27 Expanding clinical trial footprint |
Aim to understand breadth of clinical activity in solid tumors Enrolling patients globally in adaptive trial with multiple arms P Endometrial Monotherapy expansion Monotherapy IV dose escalation Checkpoint inhibitor combinations ImmTAC
combinations Chemotherapy combinations Monotherapy Combinations NSCLC Monotherapy expansion Ovarian Monotherapy expansion Cutaneous melanoma Monotherapy expansion Enables future randomized trials into earlier lines of therapy
Adaptive design enables flexible expansion size
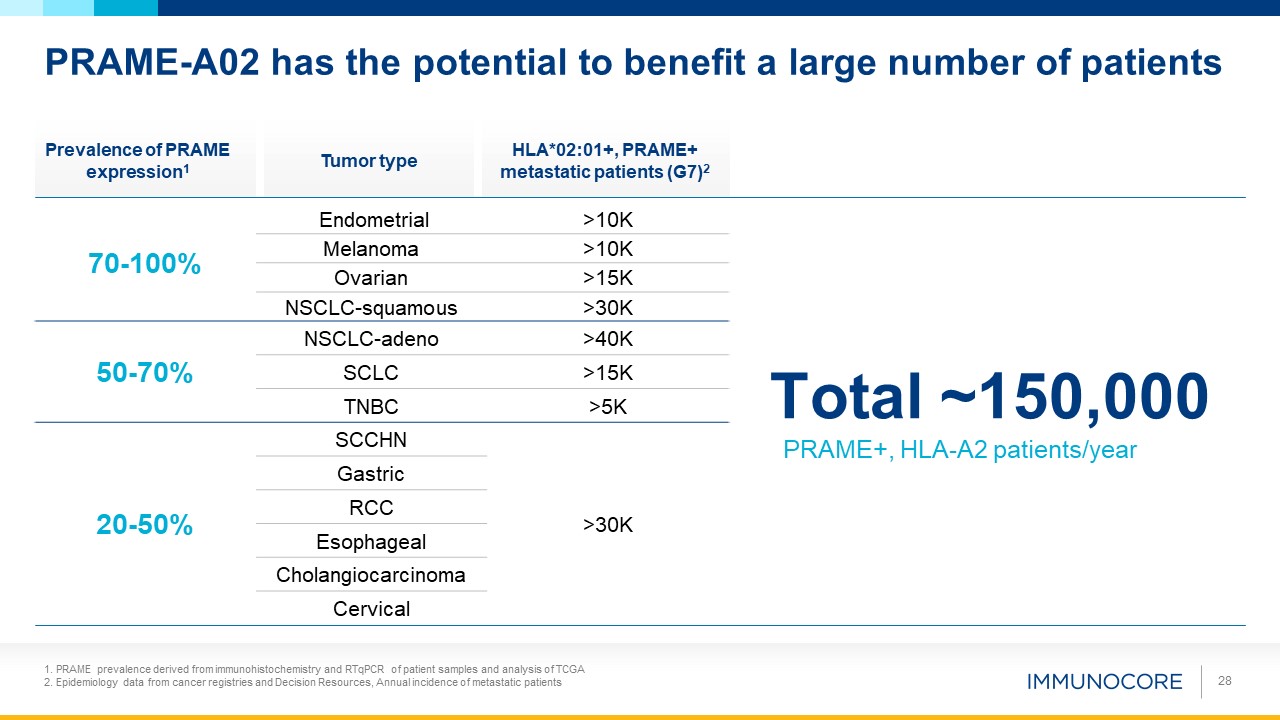
Total ~150,000 PRAME+, HLA-A2 patients/year PRAME-A02 has the potential to benefit a large number of patients Tumor
type Prevalence of PRAME expression1 HLA*02:01+, PRAME+ metastatic patients (G7)2 70-100%
Endometrial >10K Melanoma >10K Ovarian >15K NSCLC-squamous >30K 50-70% NSCLC-adeno >40K SCLC >15K TNBC >5K 20-50% SCCHN >30K Gastric RCC Esophageal Cholangiocarcinoma Cervical 28 1. PRAME
prevalence derived from immunohistochemistry and RTqPCR of patient samples and analysis of TCGA 2. Epidemiology data from cancer registries and Decision Resources, Annual incidence of metastatic patients
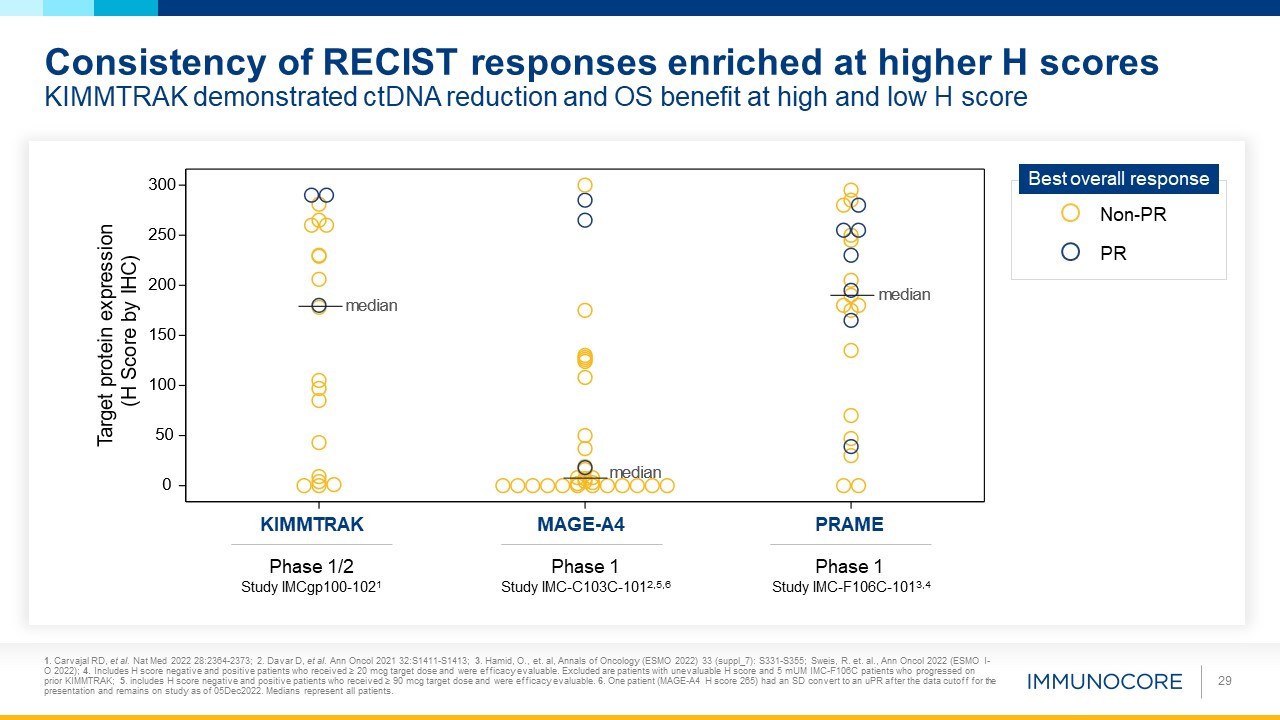
Consistency of RECIST responses enriched at higher H scoresKIMMTRAK demonstrated ctDNA reduction and OS benefit at high and low H
score 29 PRAME MAGE-A4 KIMMTRAK Phase 1/2 Study IMCgp100-1021 Phase 1 Study IMC-C103C-1012,5,6 Phase 1 Study IMC-F106C-1013,4 Non-PR PR Best overall response 1. Carvajal RD, et al. Nat Med 2022 28:2364-2373; 2. Davar D, et al.
Ann Oncol 2021 32:S1411-S1413; 3. Hamid, O., et. al, Annals of Oncology (ESMO 2022) 33 (suppl_7): S331-S355; Sweis, R. et. al., Ann Oncol 2022 (ESMO I-O 2022); 4. Includes H score negative and positive patients who received ≥ 20 mcg target dose
and were efficacy evaluable. Excluded are patients with unevaluable H score and 5 mUM IMC-F106C patients who progressed on prior KIMMTRAK; 5. includes H score negative and positive patients who received ≥ 90 mcg target dose and were efficacy
evaluable. 6. One patient (MAGE-A4 H score 265) had an SD convert to an uPR after the data cutoff for the presentation and remains on study as of 05Dec2022. Medians represent all patients.
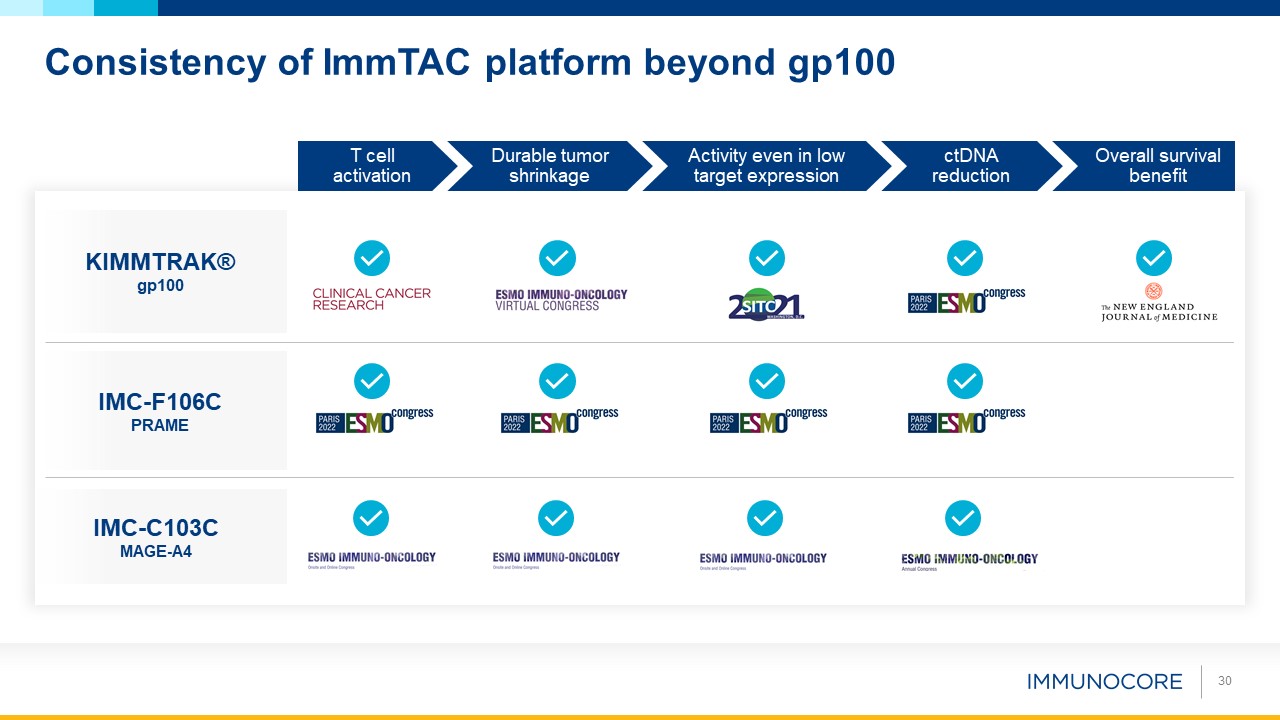
30 Consistency of ImmTAC platform beyond gp100 T cell activation Durable tumor shrinkage Activity even in low target
expression ctDNA reduction Overall survival benefit KIMMTRAK® gp100 IMC-F106C PRAME IMC-C103C MAGE-A4
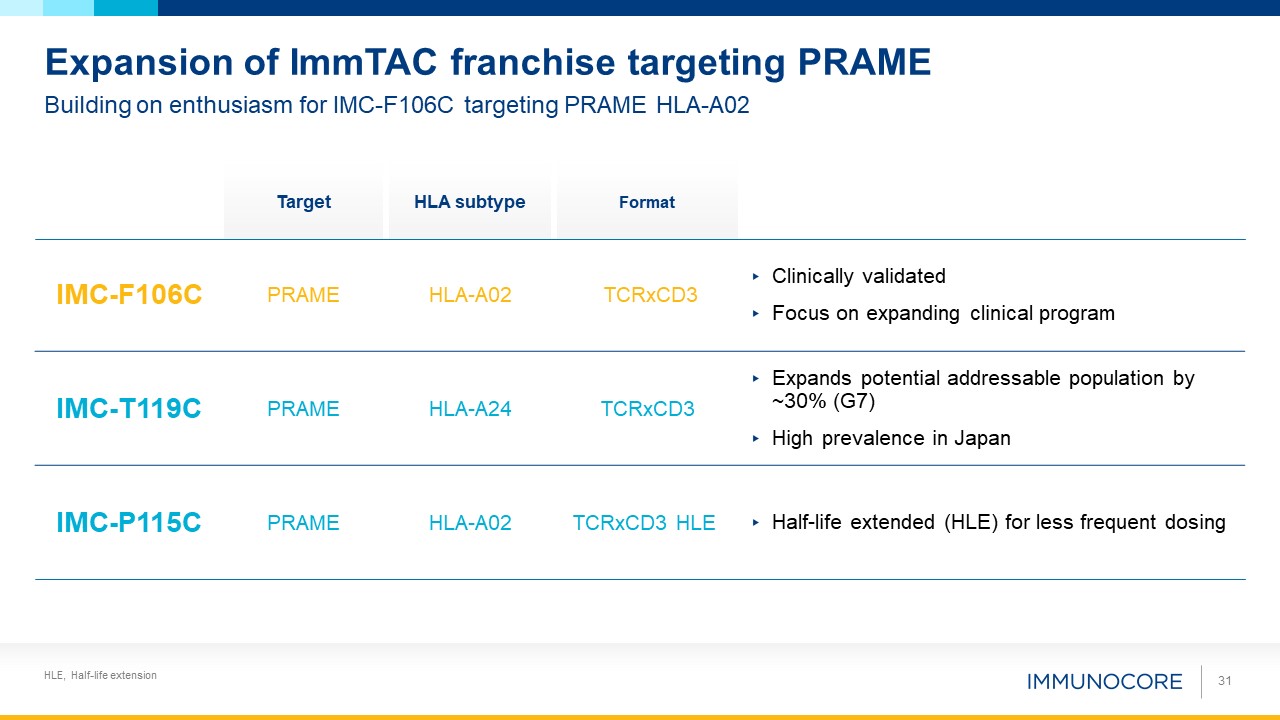
HLE, Half-life extension Building on enthusiasm for IMC-F106C targeting PRAME HLA-A02 Expansion of ImmTAC franchise targeting
PRAME IMC-F106C PRAME HLA-A02 TCRxCD3 Clinically validated Focus on expanding clinical program IMC-T119C PRAME HLA-A24 TCRxCD3 Expands potential addressable population by ~30% (G7) High prevalence in
Japan IMC-P115C PRAME HLA-A02 TCRxCD3 HLE Half-life extended (HLE) for less frequent dosing Target HLA subtype Format 31
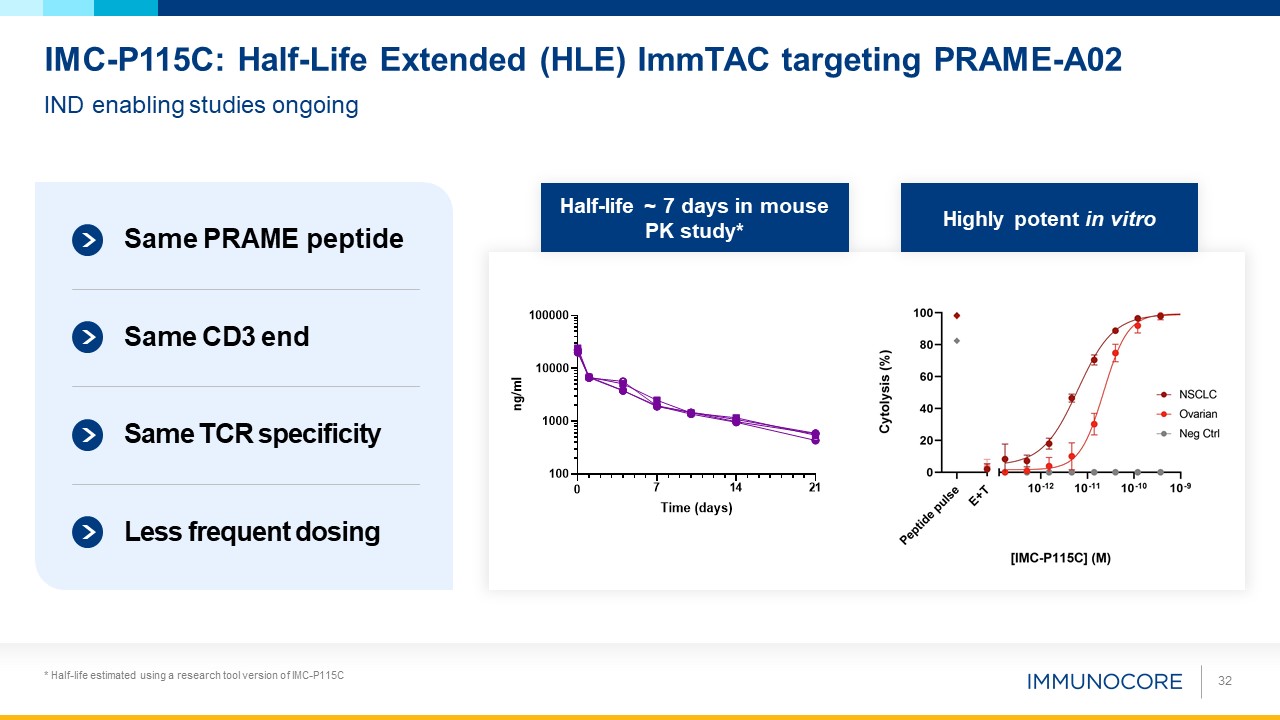
32 IND enabling studies ongoing IMC-P115C: Half-Life Extended (HLE) ImmTAC targeting PRAME-A02 * Half-life estimated using a
research tool version of IMC-P115C Same PRAME peptide Same CD3 end Less frequent dosing Same TCR specificity Half-life ~ 7 days in mouse PK study* Highly potent in vitro
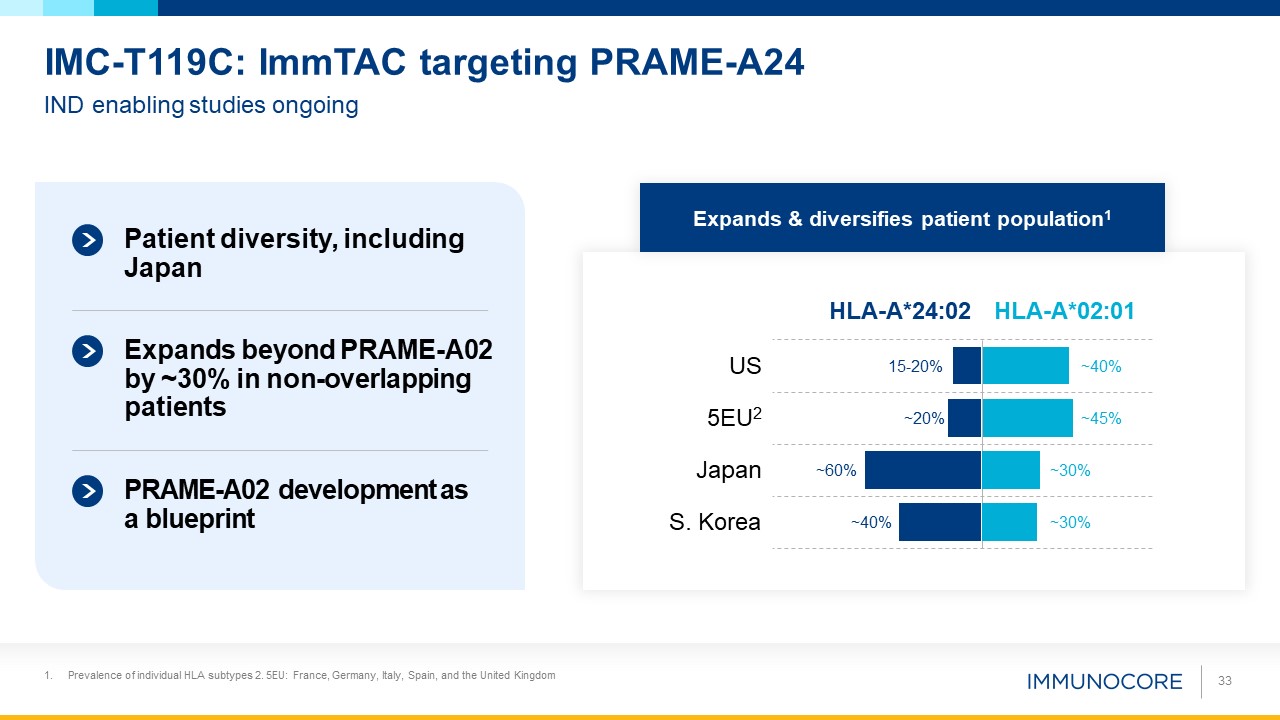
Prevalence of individual HLA subtypes 2. 5EU: France, Germany, Italy, Spain, and the United Kingdom 33 IND enabling studies
ongoing IMC-T119C: ImmTAC targeting PRAME-A24 Patient diversity, including Japan Expands beyond PRAME-A02 by ~30% in non-overlapping patients PRAME-A02 development as a
blueprint US 15-20% ~40% 5EU2 ~20% ~45% Japan ~30% ~60% HLA-A*24:02 HLA-A*02:01 S. Korea ~30% ~40% Expands & diversifies patient population1

Novel ImmTAC Candidate for GI cancers from our discovery engine
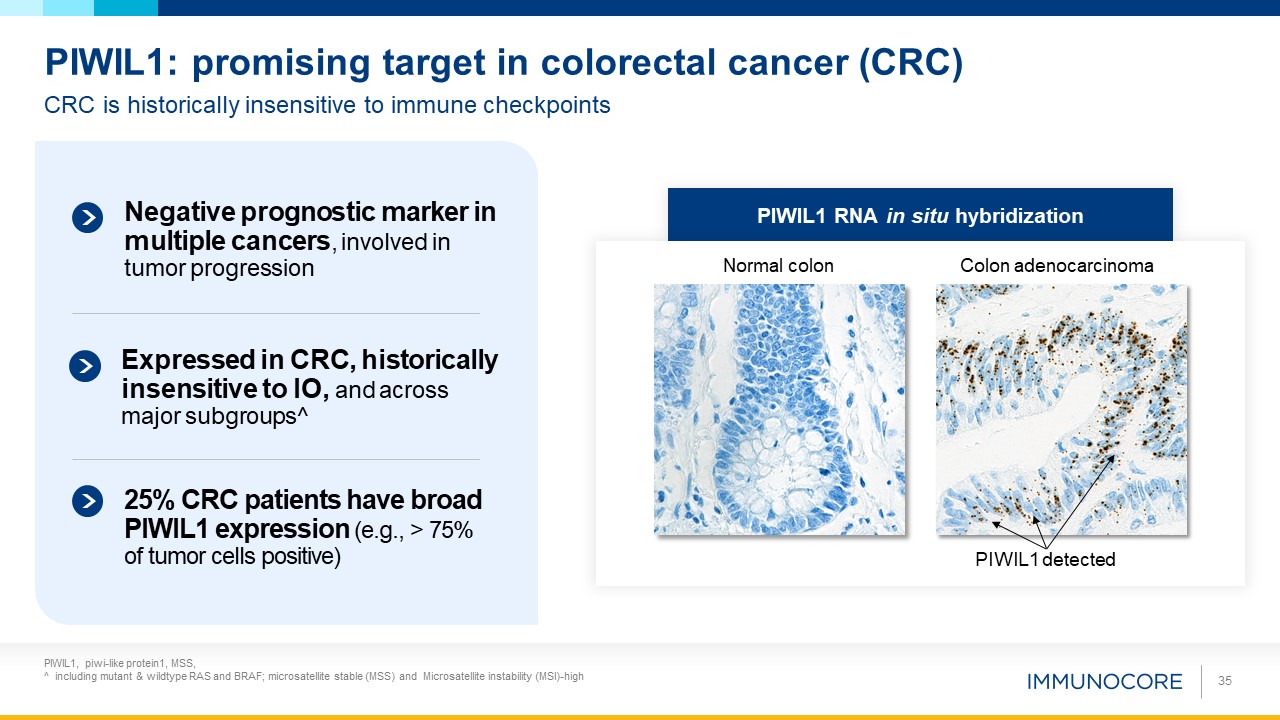
PIWIL1, piwi-like protein1, MSS, ^ including mutant & wildtype RAS and BRAF; microsatellite stable (MSS) and Microsatellite
instability (MSI)-high 35 CRC is historically insensitive to immune checkpoints PIWIL1: promising target in colorectal cancer (CRC) PIWIL1 RNA in situ hybridization Colon adenocarcinoma Normal colon PIWIL1 detected Negative
prognostic marker in multiple cancers, involved in tumor progression 25% CRC patients have broad PIWIL1 expression (e.g., > 75% of tumor cells positive) Expressed in CRC, historically insensitive to IO, and across major subgroups^

ImmTAC, Immune mobilizing T cell receptor Against Cancer; PIWIL, piwi-like protein 1 36 IND planned Q4 2023 IMC-R117C:
First-in-class immunotherapy targeting PIWIL1 (PIWIL1 x CD3) Tumor type Prevalence of PIWIL1 expression HLA*02:01+, PIWIL1+ metastatic patients
(G7) 20-30% Colorectal >20K Esophageal >xK 15-20% Ovarian ~10K Gastric >xK ~10-15% Endometroid ~6K Pancreatic >xK Total >35,000 PIWIL1+, HLA-A2 patients/year Relative PIWIL1 expression PIWIL RNA expression
Individual patient tumor n = mRNA sample size

Pursuing a functional cure in infectious diseases
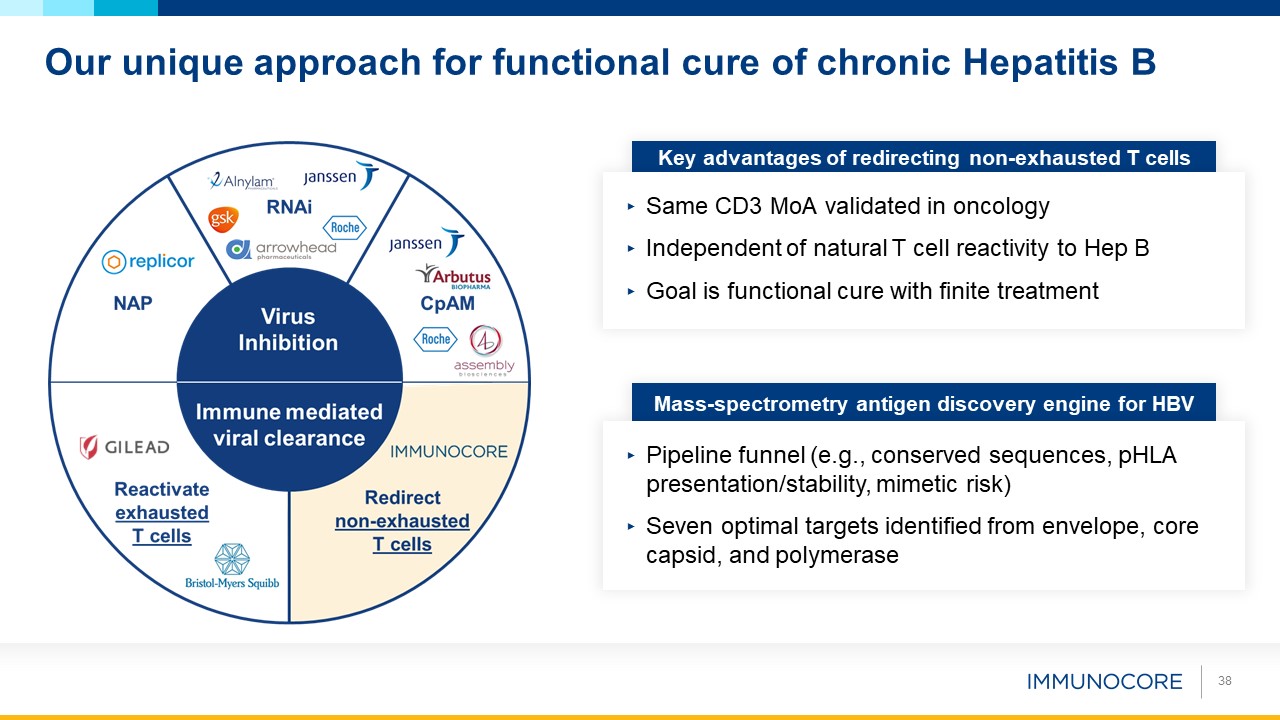
Our unique approach for functional cure of chronic Hepatitis B Key advantages of redirecting non-exhausted T cells Same CD3 MoA
validated in oncology Independent of natural T cell reactivity to Hep B Goal is functional cure with finite treatment Mass-spectrometry antigen discovery engine for HBV Pipeline funnel (e.g., conserved sequences, pHLA
presentation/stability, mimetic risk) Seven optimal targets identified from envelope, core capsid, and polymerase 38
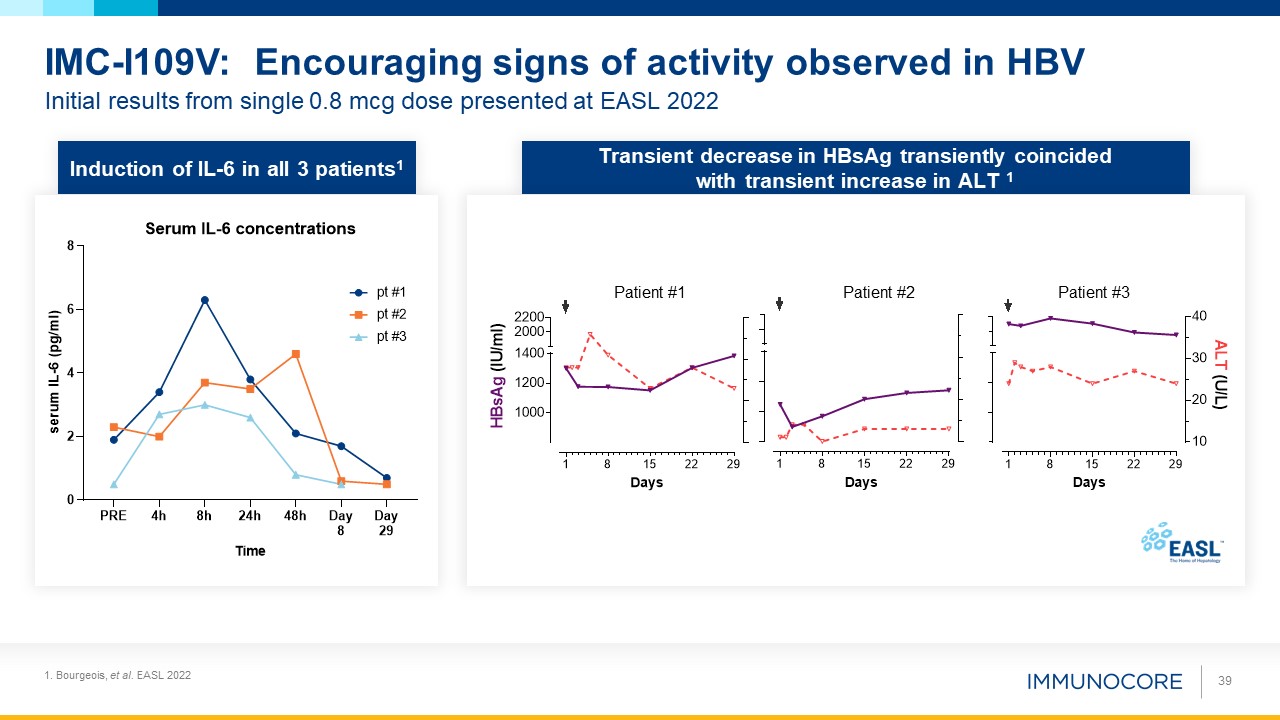
IMC-I109V: Encouraging signs of activity observed in HBV 39 Induction of IL-6 in all 3 patients1 Transient decrease in HBsAg
transiently coincided with transient increase in ALT 1 Patient #1 Patient #2 Patient #3 1. Bourgeois, et al. EASL 2022 Initial results from single 0.8 mcg dose presented at EASL 2022

40 Functional cure program for HIV with goal of eliminating HIV reservoirs Same MoA as tebentafusp, but optimized for low target
viral peptide presentation Bypasses exhausted T cells Targets highly conserved & functionally constrained viral epitopes Active in ex vivo assays of infected CD4+ T cells from ART-treated HIV patients Soluble format access to tissue
reservoirs Initial IMC-M113V Phase 1 data in 2023

KIMMTRAK commercial performance

~$400M *Preliminary financial results are approximated and unaudited. 1. “Net sales” refers to total net product and net
pre-product revenue of KIMMTRAK and tebentafusp. 2. Dollar amounts based on conversion rate of approximately 1.21. 42 ~$50M Q4 preliminary net sales of KIMMTRAK / tebentafusp1,2 Preliminary cash and cash equivalents as of December 31,
20222 YE preliminary net sales of KIMMTRAK / tebentafusp1,2 Cash runway projected into 2026 with anticipated KIMMTRAK revenues ~$140M Preliminary 2022 Financial Results

Delivering on our promise – Consistent execution

41st Annual J.P. Morgan Healthcare Conference 44 Continuing to write the next chapter of cancer and infectious diseases
treatment Looking ahead Continue responsible management of resources Sustain and grow Deliver IND for 3 new ImmTAC candidates Global site expansion for PRAME-A02 trial (data by 1H 2024) HIV Phase 1 SAD data expected 2023

THANK YOU