Exhibit 99.2

Phase 1 dose escalation of IMC-F106C, the first PRAME × CD3 ImmTAC bispecific protein in solid
tumors September 9, 2022

Forward-Looking Statements This presentation contains “forward-looking statements” within the meaning
of the Private Securities Litigation Reform Act of 1995. Words such as “may,” “can”, “will,” “believe,” “expect,” “plan,” “anticipate,” “project” and similar expressions (as well as other words or expressions referencing future events or
circumstances) are intended to identify forward-looking statements. All statements, other than statements of historical facts, included in this presentations are forward-looking statements. These statements include, but are not limited to,
statements regarding the marketing and therapeutic potential and clinical benefits of IMC-F106C for a wide range of cancers, including its ability to influence a diverse range of tumors and ability to result in a durable response; the timing
of patient enrollment for and expansion arms of the IMC- F106C-101 trial, including the option for Phase 2 expansion; and expectations regarding the development plan, design, progress, timing, scope and results of Immunocore’s existing and
planned clinical trials, including the IMC-F106C-101 trial, including statements regarding upcoming cohorts, trial expansion and the timing of the availability of future clinical trial results, the KIMMTRAK clinical development and the
marketing and therapeutic potential of KIMMTRAK for metastatic uveal melanoma (mUM), expectations regarding the potential market size and opportunity for Immunocore’s product candidates, and expectations regarding receipt of regulatory
approvals of Immunocore’s product candidates. Any forward-looking statements are based on management’s current expectations of future events and are subject to a number of risks and uncertainties that could cause actual results to differ
materially and adversely from those set forth in or implied by such forward-looking statements, many of which are beyond the Company’s control. These risks and uncertainties include, but are not limited to, the impact of the ongoing and
evolving COVID-19 pandemic on the Company’s business, strategy, clinical trials and financial position, strategy and anticipated milestones, including Immunocore’s ability to conduct ongoing and planned clinical trials; Immunocore’s ability
to obtain a clinical supply of current or future product candidates, including IMC-F106C, or commercial supply of KIMMTRAK or any future approved product, including as a result of the COVID-19 pandemic, war in Ukraine or global geopolitical
tension; Immunocore’s ability to obtain and maintain regulatory approvals for its product candidates, including KIMMTRAK and IMC-F106C; its ability to develop, manufacture and commercialize IMC-F106C and its other product candidates;
Immunocore’s ability and plans in continuing to establish and expand a commercial infrastructure and to successfully launch, market and sell KIMMTRAK and any future approved products; the delay of the IMC-F106C-101 trial or any other current
or planned clinical trials, whether due to the COVID-19 pandemic, patient enrollment delays or otherwise; Immunocore’s ability to successfully demonstrate the safety and efficacy of its product candidates and gain approval of its product
candidates on a timely basis, if at all; actions of regulatory agencies, which may affect the initiation, timing and progress of the IMC-F106C-101 trial and Immunocore’s other clinical trials or future regulatory approval; Immunocore’s need
for and ability to obtain additional funding, on favorable terms or at all, including as a result of rising inflation, interest rates and general market conditions, and the impacts thereon of the COVID-19 pandemic, war in Ukraine and global
geopolitical tension; Immunocore’s ability to obtain, maintain and enforce intellectual property protection for KIMMTRAK or any product candidates it is developing; unexpected safety or efficacy data observed during preclinical studies or
clinical trials, including the IMC-F106C-101 trial; clinical trial site activation or enrollment rates that are lower than expected; changes in expected or existing competition; and the success of Immunocore’s current and future
collaborations, partnerships or licensing arrangements. These and other risks and uncertainties are described in greater detail in the section titled "Risk Factors" in Immunocore’s filings with the Securities and Exchange Commission,
including Immunocore’s most recent Annual Report on Form 20-F for the year ended December 31, 2021 filed with the Securities and Exchange Commission on March 3, 2022, as well as discussions of potential risks, uncertainties, and other
important factors in the Company’s subsequent filings with the Securities and Exchange Commission. All information in this presentation is as of the date of the release, and the Company undertakes no duty to update this information, except as
required by law. Such risks may be amplified by the COVID-19 pandemic, war in Ukraine and related geopolitical tension, and their potential impacts on Immunocore’s business and the overall global economy. All forward-looking statements
contained in this presentation speak only as of the date on which they were made and should not be relied upon as representing its views as of any subsequent date. Except to the extent required by law, Immunocore undertakes no obligation to
update such statements to reflect events that occur or circumstances that exist after the date on which they were made. This presentation contains non-IFRS financial measures, including Adjusted Cash and Cash Equivalents, which have certain
limitations and should not be considered in isolation, or as alternatives or substitutes for, financial measures determined in accordance with IFRS. Certain information contained in this presentation relates to or is based on studies,
publications, surveys, and other data obtained from third-party sources and Immunocore’s own internal estimates and research. While Immunocore believes these third-party sources to be reliable as of the date of this presentation, it has not
independently verified, and makes no representation as to the adequacy, fairness, accuracy, or completeness of, any information obtained from third-party sources. KIMMTRAK™ is a trademark owned or licensed to Immunocore. 2

3 Overview & ImmTAC Platform Bahija Jallal, PhD – Chief Executive Officer Phase 1 study of
IMC-F106C Targeting PRAME Omid Hamid, MD – Cedars-Sinai Cancer, the Angeles Clinic & Research Institute Next steps for IMC-F106C David Berman, MD, PhD – Head of R&D Concluding Remarks Bahija Jallal, PhD – Chief Executive
Officer Q&A Session
4 We are defining a new frontier of cancer treatment T Cell Receptor (TCR) Therapy Off-the-shelf
bispecific T cell engagers 1949 Targeted Therapy Chemotherapy 1997 Immunotherapy 2011 Antibody-Drug Conjugate 2013 Cell Therapy 2017 2022

5 Omid Hamid, MD Chief, Translational Research and Immunotherapy and Co-Director, Melanoma
Therapeutics Internationally recognized leader in immuno-oncology drug development and melanoma therapeutics Investigator in the initial trials with ipilimumab, pembrolizumab, nivolumab, atezolizumab and vemurafenib Current focus on
next-generation checkpoint inhibitors, T cell adoptive therapies and bispecific antibodies

Phase 1 dose escalation of IMC-F106C, the first PRAME × CD3 ImmTAC bispecific protein in solid
tumors Omid Hamid,1 Takami Sato,2 Diwakar Davar,3 Margaret Callahan,4 Fiona Thistlethwaite,5 Raid Aljumaily,6 Melissa Johnson,7 Hendrik-Tobias Arkenau,8 Ecaterina Dumbrava,9 Benjamin Izar,10 Hui Amy Chen,11 Shannon Marshall,12 Yuan Yuan,12
Mugdha Deo,12 Sarah Stanhope,12 Laura Collins,12 Renee Mundy,12 Shaad Abdullah,12 Juanita Lopez13 1The Angeles Clinic and Research Institute, A Cedars-Sinai Affiliate, Los Angeles, CA, US; 2Thomas Jefferson University Hospital, Philadelphia,
PA, US; 3UPMC Hillman Cancer Center, Pittsburgh, PA, US; 4Memorial Sloan Kettering Cancer Center, New York, NY, US; 5The Christie NHS Foundation Trust and University of Manchester, Manchester, UK; 6University of Oklahoma Peggy and Charles
Stephenson Cancer Center, Oklahoma City, OK, US; 7Sarah Cannon Research Institute, Nashville, TN, US; 8Sarah Cannon Research Institute, London, UK; 9MD Anderson Cancer Center, Houston, TX, US; 10Columbia University Medical Center, New York,
NY, US; 11University of California Davis Comprehensive Cancer Center, Sacramento, CA, US; 12Immunocore Ltd, Abingdon, UK; 13The Royal Marsden NHS Foundation Trust and Institute of Cancer Research, Sutton, UK #728O

Content of this presentation is copyright and responsibility of the author. Permission is required for
re-use. DECLARATION OF INTERESTS 2 Dr Omid Hamid Advisory/Consulting: Aduro Biotech, Akeso Biopharma, Alkermes, Amgen, BeiGene, BioAtla, BMS, Genentech, GlaxoSmithKline, Idera, Immunocore, Incyte, Iovance Biotherapeutics, Janssen, Merck,
NextCure, Novartis, Pfizer, Regeneron, Roche, Sanofi, Seattle Genetics, Tempus, Zelluna; Speaker’s Bureau: BMS, Novartis, Pfizer, Sanofi/Regeneron Honoraria: BMS, Novartis, Pfizer, Sanofi/Regeneron Research Funding (Institute): Aduro
Biotech, Akeso Biopharma, Amgen, Arcus Biosciences, Bioatla, BMS, CytomX Therapeutics, Exelixis, Genentech, GlaxoSmithKline, Idera, Immunocore, Incyte, Iovance Biotherapeutics, Merck, Merck Serono, Moderna Therapeutics, NextCure, Novartis,
Pfizer, Regeneron, Roche, Rubius Therapeutics, Sanofi, Seattle Genetics, Torque, Zelluna DISCLAIMER All statements contained in this presentation are based on preclinical and clinical trial data related to an investigational molecule,
IMC-F106C. Development of this molecule is ongoing and, therefore, statements relating to study data to date should not be regarded as definitive reflections of safety, efficacy or the risk-benefit profile of the molecule.
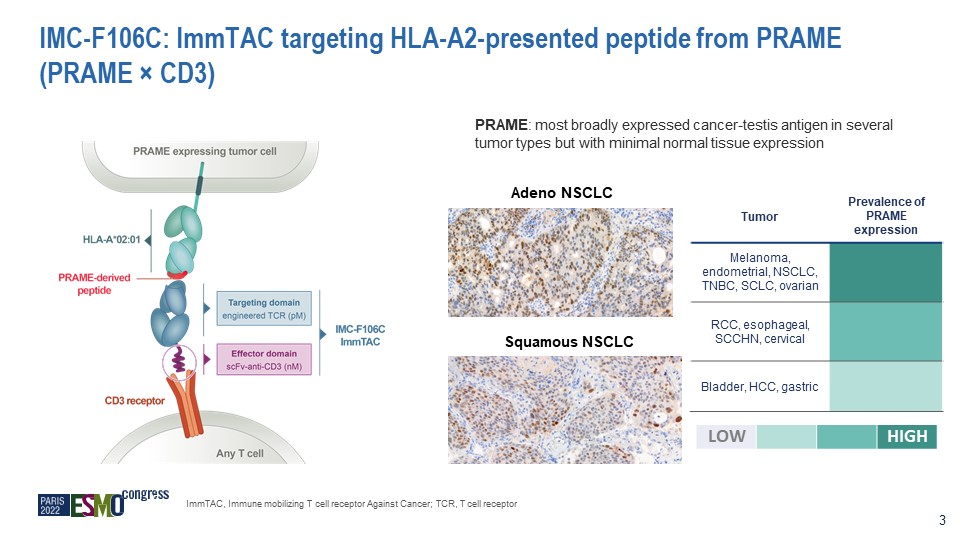
IMC-F106C: ImmTAC targeting HLA-A2-presented peptide from PRAME (PRAME × CD3) PRAME: most broadly
expressed cancer-testis antigen in several tumor types but with minimal normal tissue expression Adeno NSCLC Squamous NSCLC Tumor Prevalence of PRAME expression Melanoma, endometrial, NSCLC, TNBC, SCLC, ovarian RCC, esophageal, SCCHN,
cervical Bladder, HCC, gastric LOW HIGH ImmTAC, Immune mobilizing T cell receptor Against Cancer; TCR, T cell receptor 3
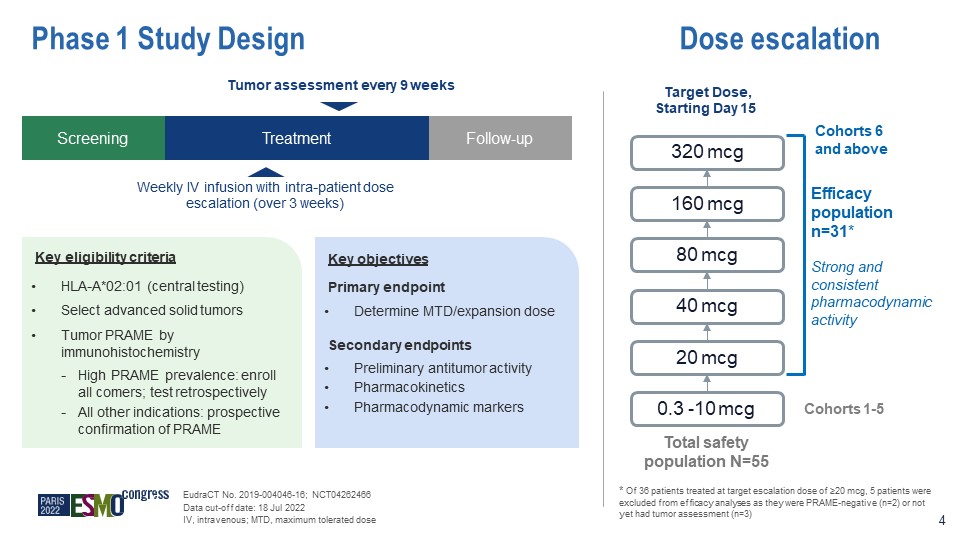
Phase 1 Study Design Key objectives Primary endpoint Determine MTD/expansion dose Secondary
endpoints Preliminary antitumor activity Pharmacokinetics Pharmacodynamic markers Key eligibility criteria HLA-A*02:01 (central testing) Select advanced solid tumors Tumor PRAME by immunohistochemistry High PRAME prevalence: enroll
all comers; test retrospectively All other indications: prospective confirmation of PRAME Screening Treatment Follow-up Weekly IV infusion with intra-patient dose escalation (over 3 weeks) Tumor assessment every 9 weeks 0.3 -10 mcg 20
mcg 40 mcg 80 mcg 160 mcg 320 mcg Efficacy population n=31* Strong and consistent pharmacodynamic activity Total safety population N=55 * Of 36 patients treated at target escalation dose of ≥20 mcg, 5 patients were excluded from
efficacy analyses as they were PRAME-negative (n=2) or not yet had tumor assessment (n=3) EudraCT No. 2019-004046-16; NCT04262466 Data cut-off date: 18 Jul 2022 IV, intravenous; MTD, maximum tolerated dose Dose escalation Target Dose,
Starting Day 15 Cohorts 1-5 4 Cohorts 6 and above
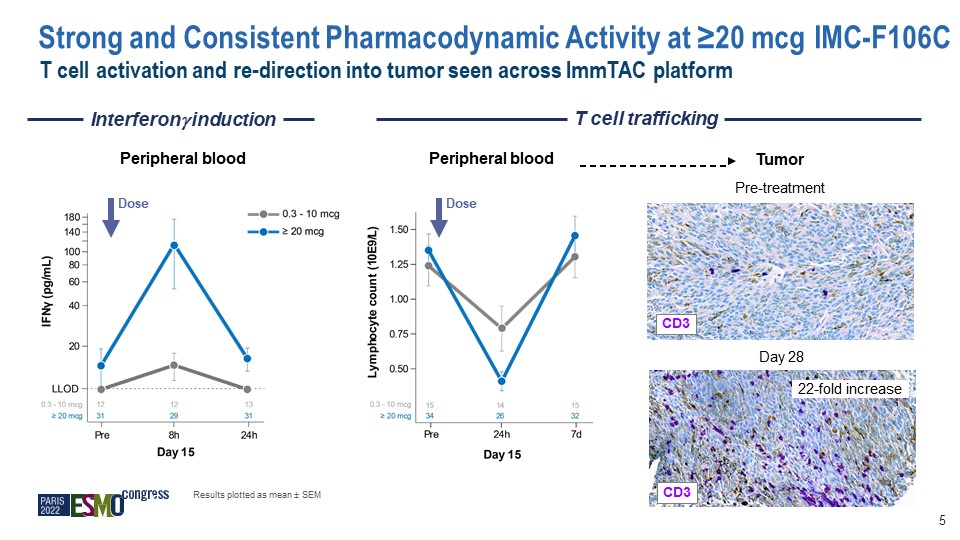
Interferon induction T cell trafficking Peripheral blood Peripheral blood Tumor Pre-treatment Day
28 CD3 22-fold increase Dose Dose Results plotted as mean ± SEM 5 CD3 T cell activation and re-direction into tumor seen across ImmTAC platform Strong and Consistent Pharmacodynamic Activity at ≥20 mcg IMC-F106C
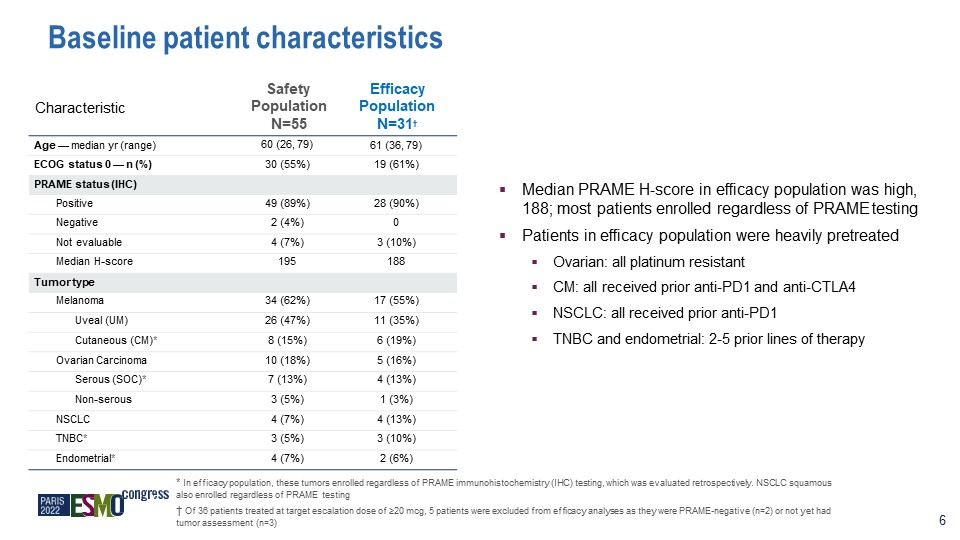
Characteristic Safety Population N=55 Efficacy Population N=31† Age — median yr (range) 60 (26,
79) 61 (36, 79) ECOG status 0 — n (%) 30 (55%) 19 (61%) PRAME status (IHC) Positive 49 (89%) 28 (90%) Negative 2 (4%) 0 Not evaluable 4 (7%) 3 (10%) Median H-score 195 188 Tumor type Melanoma 34 (62%) 17 (55%) Uveal
(UM) 26 (47%) 11 (35%) Cutaneous (CM)* 8 (15%) 6 (19%) Ovarian Carcinoma 10 (18%) 5 (16%) Serous (SOC)* 7 (13%) 4 (13%) Non-serous 3 (5%) 1 (3%) NSCLC 4 (7%) 4 (13%) TNBC* 3 (5%) 3 (10%) Endometrial* 4 (7%) 2
(6%) Baseline patient characteristics 6 Median PRAME H-score in efficacy population was high, 188; most patients enrolled regardless of PRAME testing Patients in efficacy population were heavily pretreated Ovarian: all platinum
resistant CM: all received prior anti-PD1 and anti-CTLA4 NSCLC: all received prior anti-PD1 TNBC and endometrial: 2-5 prior lines of therapy * In efficacy population, these tumors enrolled regardless of PRAME immunohistochemistry (IHC)
testing, which was evaluated retrospectively. NSCLC squamous also enrolled regardless of PRAME testing † Of 36 patients treated at target escalation dose of ≥20 mcg, 5 patients were excluded from efficacy analyses as they were PRAME-negative
(n=2) or not yet had tumor assessment (n=3)
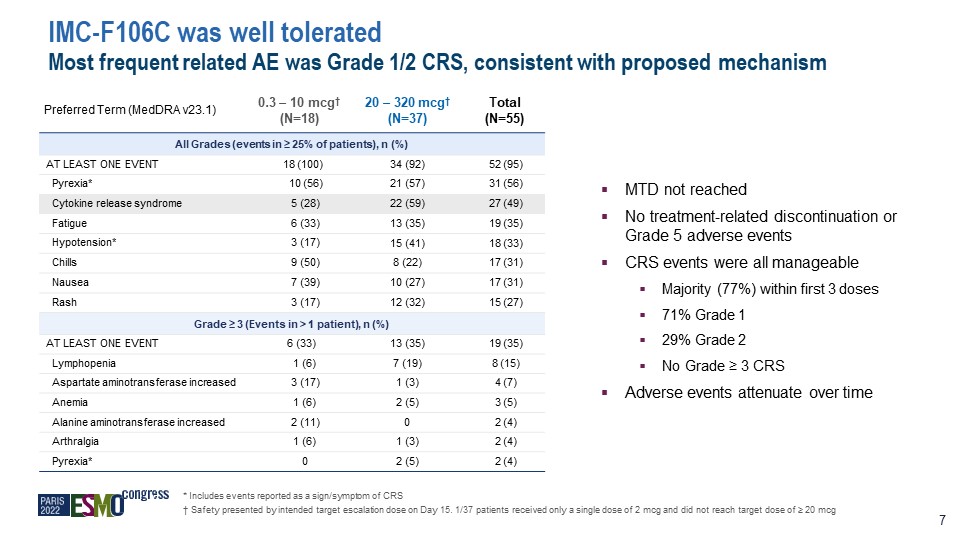
IMC-F106C was well tolerated Most frequent related AE was Grade 1/2 CRS, consistent with proposed
mechanism Preferred Term (MedDRA v23.1) 0.3 – 10 mcg† (N=18) 20 – 320 mcg† (N=37) Total (N=55) All Grades (events in ≥ 25% of patients), n (%) AT LEAST ONE EVENT 18 (100) 34 (92) 52 (95) Pyrexia* 10 (56) 21 (57) 31
(56) Cytokine release syndrome 5 (28) 22 (59) 27 (49) Fatigue 6 (33) 13 (35) 19 (35) Hypotension* 3 (17) 15 (41) 18 (33) Chills 9 (50) 8 (22) 17 (31) Nausea 7 (39) 10 (27) 17 (31) Rash 3 (17) 12 (32) 15 (27) Grade ≥ 3
(Events in > 1 patient), n (%) AT LEAST ONE EVENT 6 (33) 13 (35) 19 (35) Lymphopenia 1 (6) 7 (19) 8 (15) Aspartate aminotransferase increased 3 (17) 1 (3) 4 (7) Anemia 1 (6) 2 (5) 3 (5) Alanine aminotransferase
increased 2 (11) 0 2 (4) Arthralgia 1 (6) 1 (3) 2 (4) Pyrexia* 0 2 (5) 2 (4) MTD not reached No treatment-related discontinuation or Grade 5 adverse events CRS events were all manageable Majority (77%) within first 3
doses 71% Grade 1 29% Grade 2 No Grade ≥ 3 CRS Adverse events attenuate over time * Includes events reported as a sign/symptom of CRS † Safety presented by intended target escalation dose on Day 15. 1/37 patients received only a single
dose of 2 mcg and did not reach target dose of ≥ 20 mcg 7
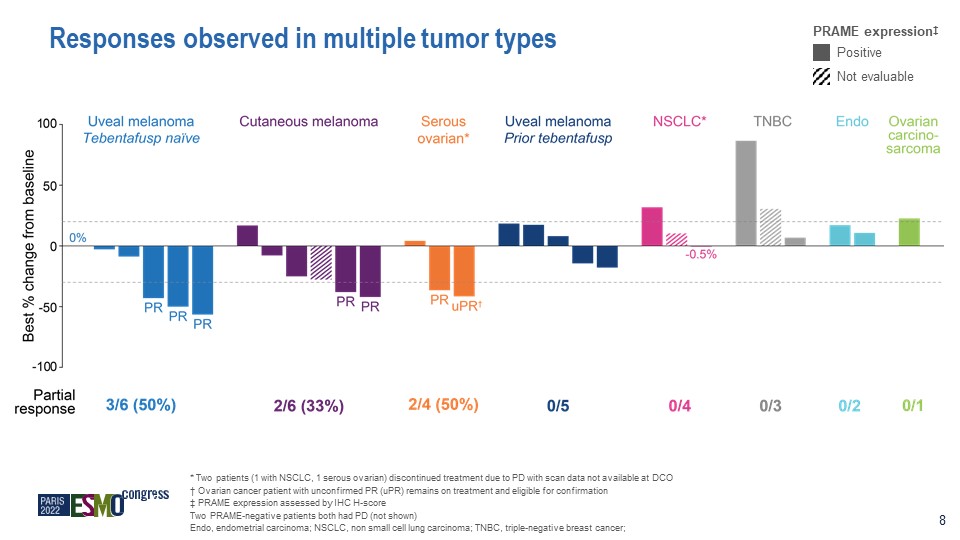
Responses observed in multiple tumor types * Two patients (1 with NSCLC, 1 serous ovarian)
discontinued treatment due to PD with scan data not available at DCO † Ovarian cancer patient with unconfirmed PR (uPR) remains on treatment and eligible for confirmation ‡ PRAME expression assessed by IHC H-score Two PRAME-negative
patients both had PD (not shown) Endo, endometrial carcinoma; NSCLC, non small cell lung carcinoma; TNBC, triple-negative breast cancer; PRAME expression‡ Positive Not evaluable 8
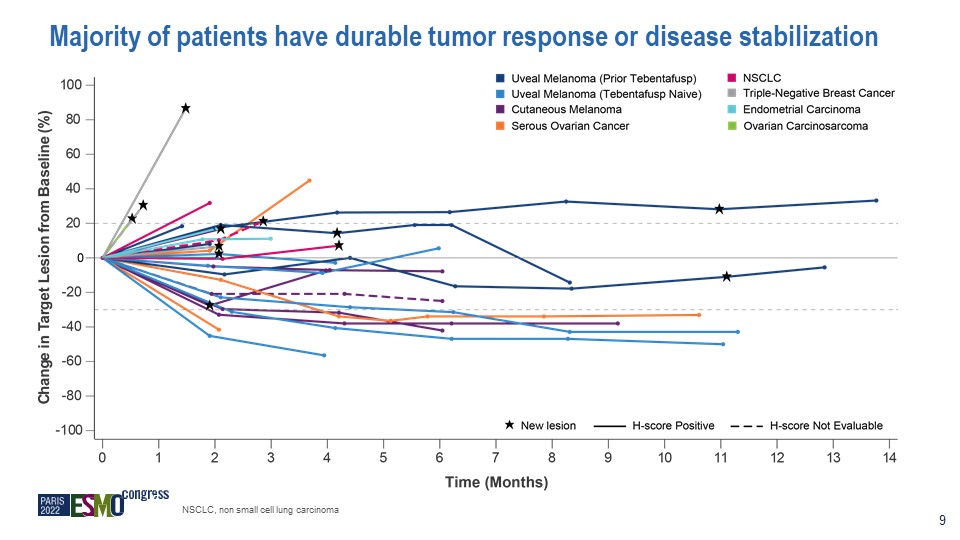
Majority of patients have durable tumor response or disease stabilization 9 NSCLC, non small cell
lung carcinoma
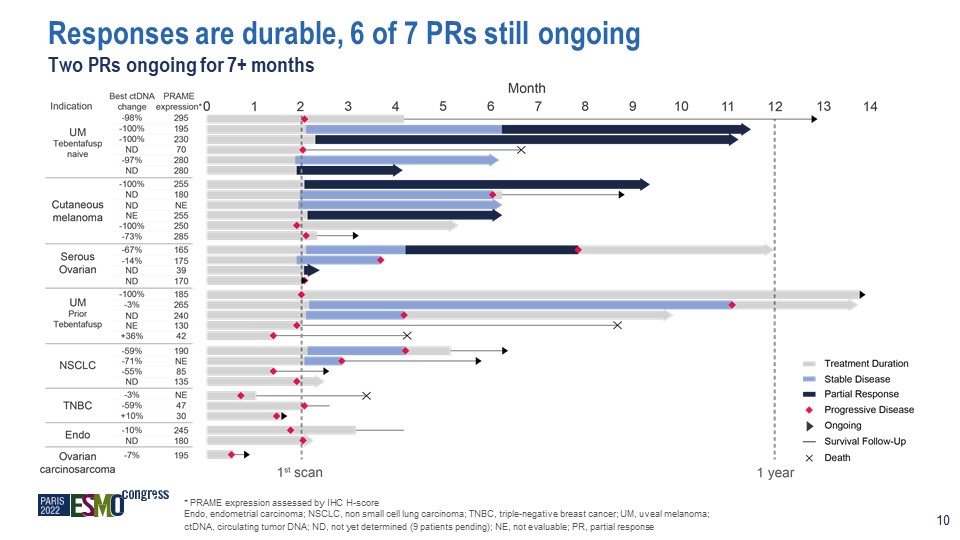
* PRAME expression assessed by IHC H-score Endo, endometrial carcinoma; NSCLC, non small cell lung
carcinoma; TNBC, triple-negative breast cancer; UM, uveal melanoma; ctDNA, circulating tumor DNA; ND, not yet determined (9 patients pending); NE, not evaluable; PR, partial response Responses are durable, 6 of 7 PRs still ongoing Two PRs
ongoing for 7+ months 10
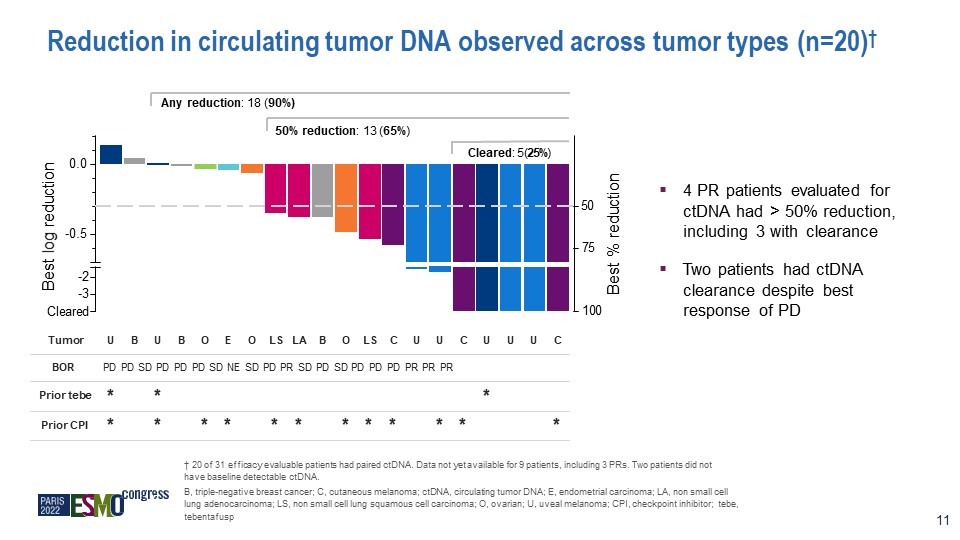
Reduction in circulating tumor DNA observed across tumor types (n=20)† Tumor U B U B O E O LS LA B O
LS C U U C U U U C BOR Prior tebe Prior CPI PD PD SD PD PD PD SD NE SD PD PR SD PD SD PD PD PD PR PR PR * * * * * * * * * * * * * * * † 20 of 31 efficacy evaluable patients had paired ctDNA. Data not yet available for 9 patients,
including 3 PRs. Two patients did not have baseline detectable ctDNA. B, triple-negative breast cancer; C, cutaneous melanoma; ctDNA, circulating tumor DNA; E, endometrial carcinoma; LA, non small cell lung adenocarcinoma; LS, non small cell
lung squamous cell carcinoma; O, ovarian; U, uveal melanoma; CPI, checkpoint inhibitor; tebe, tebentafusp Any reduction: 18 (90%) 50% reduction: 13 (65%) Cleared: 5 (25%) -0.5 0.0 Best log reduction Best %
reduction -2 -3 Cleared 50 100 75 11 4 PR patients evaluated for ctDNA had > 50% reduction, including 3 with clearance Two patients had ctDNA clearance despite best response of PD

Baseline On treatment Example responders: ovarian carcinoma and uveal melanoma Images courtesy of
Dr. Marlana Orloff (TJU) and Dr. Anja Williams (SCRI-UK) Confirmed PR ctDNA cleared Ongoing treatment 1+ year Week 18 Uveal Melanoma Unconfirmed PR Ongoing treatment; ctDNA pending Representative lesion Week 9 12 Patient #1 Ovarian
cancer 5 prior lines, platinum resistant Patient #2
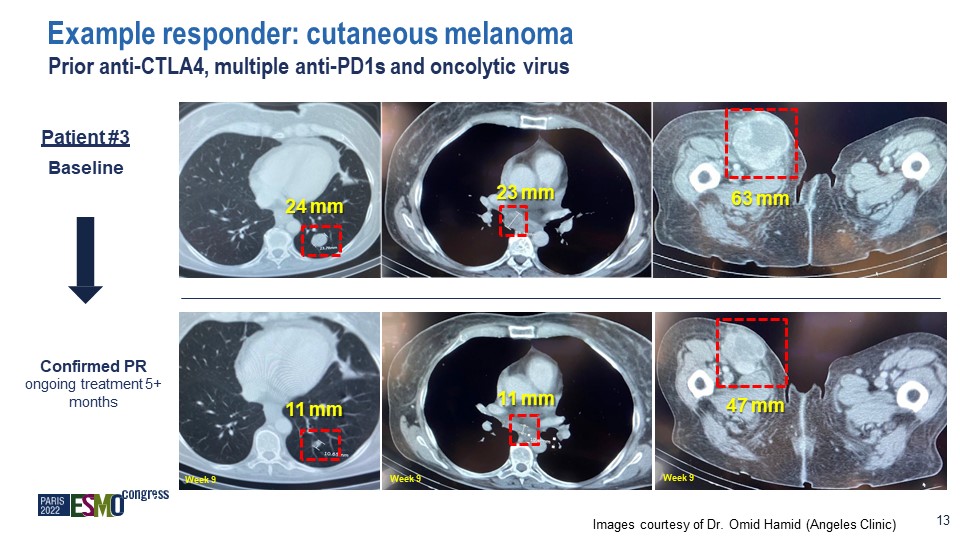
Images courtesy of Dr. Omid Hamid (Angeles Clinic) 13 Confirmed PR ongoing treatment 5+ months Week
9 Week 9 Week 9 Example responder: cutaneous melanoma Prior anti-CTLA4, multiple anti-PD1s and oncolytic virus 24 mm 11 mm 23 mm 11 mm 63 mm 47 mm Patient #3 Baseline
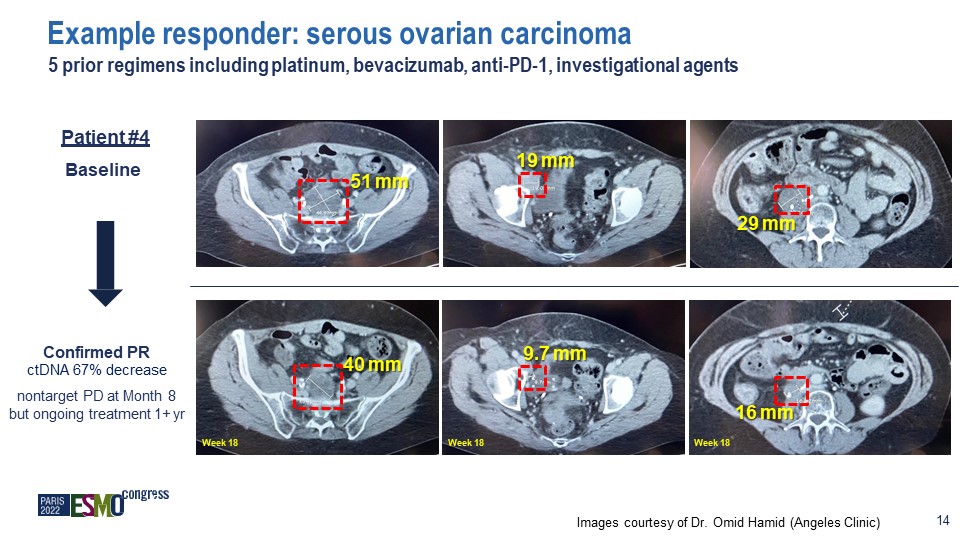
Baseline Images courtesy of Dr. Omid Hamid (Angeles Clinic) 14 Confirmed PR ctDNA 67%
decrease nontarget PD at Month 8 but ongoing treatment 1+ yr Example responder: serous ovarian carcinoma 5 prior regimens including platinum, bevacizumab, anti-PD-1, investigational agents 12 51 mm 40 mm 9.7 mm 19 mm 29 mm 16
mm Week 18 Week 18 Week 18 Patient #4
Conclusions IMC-F106C, first PRAME×CD3 ImmTAC, activates T cells and is well-tolerated CRS is mostly
Grade 1, no Grade ≥3, and predominantly during initial 3 doses Treatment-related AEs are manageable; none have led to discontinuation or death Durable (up to 9+ months) RECIST PRs across multiple tumor types, including Cutaneous melanoma,
progressed following prior anti-PD1 and anti-CTLA4 Heavily pre-treated, platinum-resistant ovarian carcinoma Uveal melanoma Benefit also apparent in disease control, including conversion of SD to PR Almost all evaluable patients, across
multiple tumor types, have ctDNA reduction Early reduction appears associated with clinical benefit Complete ctDNA clearance common in melanoma Expansions open in cutaneous melanoma, NSCLC, endometrial and ovarian carcinoma Dose
escalation continues and combinations with chemotherapy and checkpoint inhibitors planned 15

Thank you to all patients, their families and their caregivers who were involved in this global
clinical trial & all investigators and their teams Omid Hamid Takami Sato and Marlana Orloff Diwakar Davar Margaret Callahan Raid Aljumaily Melissa Johnson Ecaterina Dumbrava Benjamin Izar Hui Amy Chen The Angeles Clinic and Research
Institute, A Cedars-Sinai Affiliate Thomas Jefferson University Hospitals University of Pittsburgh Medical Center Memorial Sloan Kettering Cancer Center University of Oklahoma Peggy and Charles Stephenson Cancer Center Sarah Cannon Research
Institute, Nashville MD Anderson Cancer Center Columbia University Medical Center University of California Davis Comprehensive Cancer Center Juanita Lopez Royal Marsden NHS Foundation Trust and Institute of Cancer Research Anja Williams
and Hendrik-Tobias Arkenau Sarah Cannon Research Institute, London Fiona Thistlethwaite Heather Shaw The Christie NHS Foundation Trust University College London

22 IMC-F106C Clinical Development Plan DAVID BERMAN Head of Research and Development
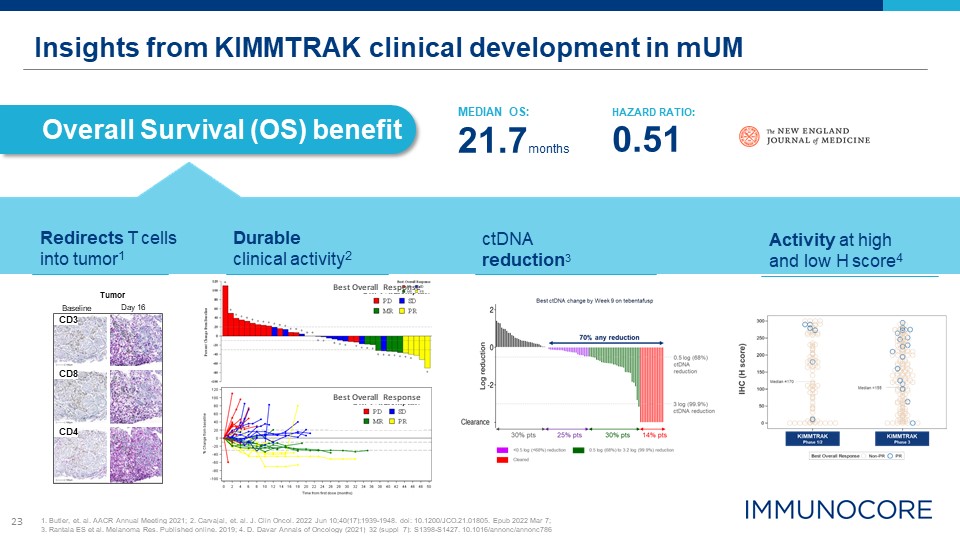
23 Insights from KIMMTRAK clinical development in mUM Overall Survival (OS) benefit Tumor Day
16 Baseline CD8 CD4 CD3 MEDIAN OS: 21.7months HAZARD RATIO: 0.51 Redirects T cells into tumor1 Durable clinical activity2 Activity at high and low H score4 1. Butler, et. al. AACR Annual Meeting 2021; 2. Carvajal, et. al. J. Clin
Oncol. 2022 Jun 10;40(17):1939-1948. doi: 10.1200/JCO.21.01805. Epub 2022 Mar 7; 3. Rantala ES et al. Melanoma Res. Published online. 2019; 4. D. Davar Annals of Oncology (2021) 32 (suppl_7): S1398-S1427. 10.1016/annonc/annonc786 Best
Overall Response Best Overall Response ctDNA reduction3
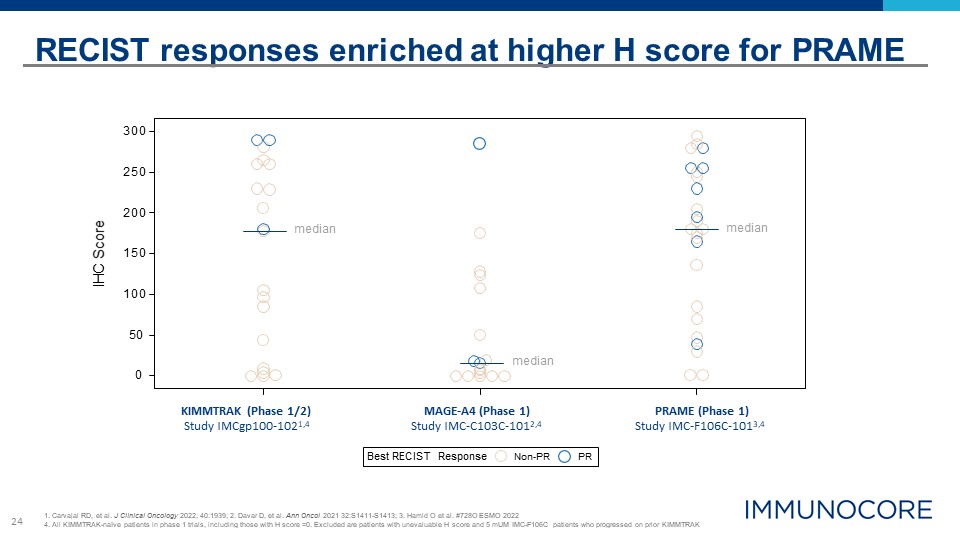
24 Best RECIST Response Non-PR PR 0 150 100 50 300 250 200 IHC Score RECIST responses
enriched at higher H score for PRAME 1. Carvajal RD, et al. J Clinical Oncology 2022; 40:1939; 2. Davar D, et al. Ann Oncol 2021 32:S1411-S1413; 3. Hamid O et al. #728O ESMO 2022 4. All KIMMTRAK-naïve patients in phase 1 trials, including
those with H score =0. Excluded are patients with unevaluable H score and 5 mUM IMC-F106C patients who progressed on prior KIMMTRAK KIMMTRAK (Phase 1/2) Study IMCgp100-1021,4 MAGE-A4 (Phase 1) Study IMC-C103C-1012,4 PRAME (Phase
1) Study IMC-F106C-1013,4 median median median
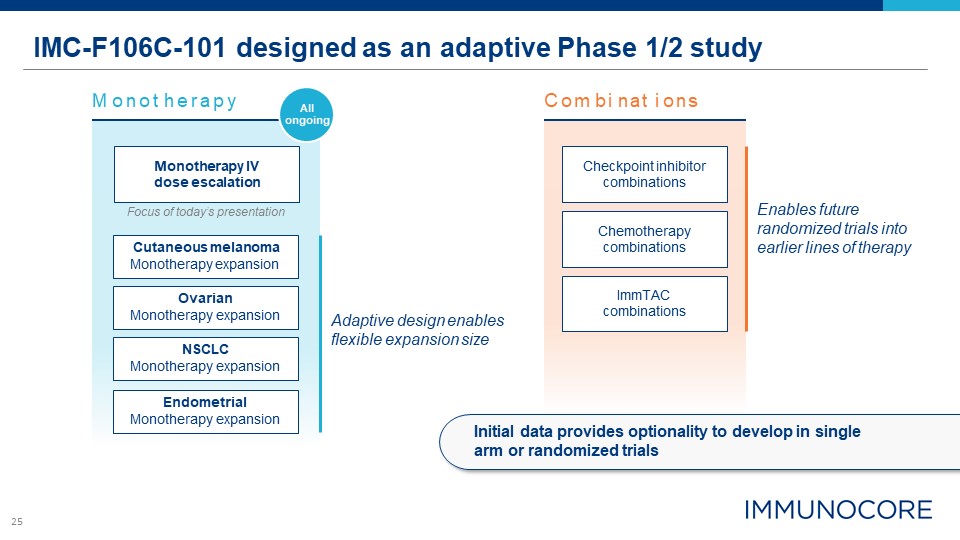
25 IMC-F106C-101 designed as an adaptive Phase 1/2 study Endometrial Monotherapy
expansion Monotherapy IV dose escalation Focus of today’s presentation Checkpoint inhibitor combinations ImmTAC combinations Chemotherapy combinations M onot herapy C om bi nat i ons NSCLC Monotherapy expansion Ovarian Monotherapy
expansion Cutaneous melanoma Monotherapy expansion Enables future randomized trials into earlier lines of therapy Adaptive design enables flexible expansion size All ongoing Initial data provides optionality to develop in single arm or
randomized trials
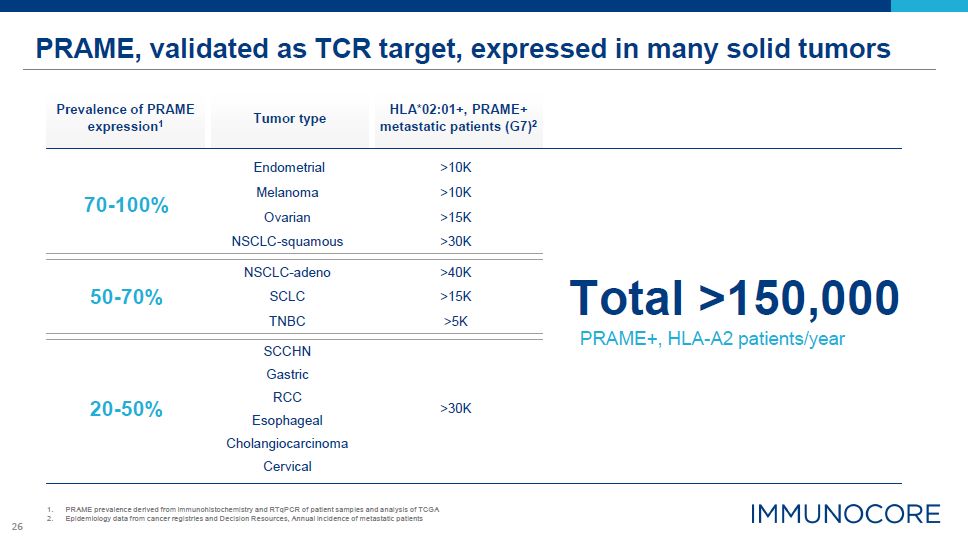
26 Total >150,000 PRAME, validated as TCR target, expressed in many solid tumors PRAME prevalence
derived from immunohistochemistry and RTqPCR of patient samples and analysis of TCGA Epidemiology data from cancer registries and Decision Resources, Annual incidence of metastatic patients Tumor type Prevalence of PRAME
expression1 HLA*02:01+, PRAME+ metastatic patients (G7)2 Endometrial >10K 70-100% Melanoma >10K Ovarian >15K NSCLC-squamous >30K 50-70% NSCLC-adeno SCLC TNBC >40K >15K >5K SCCHN PRAME+, HLA-A2
patients/year Gastric 20-50% RCC >30K Esophageal Cholangiocarcinoma Cervical

27 Concluding Remarks BAHIJA JALLAL Chief Executive Officer
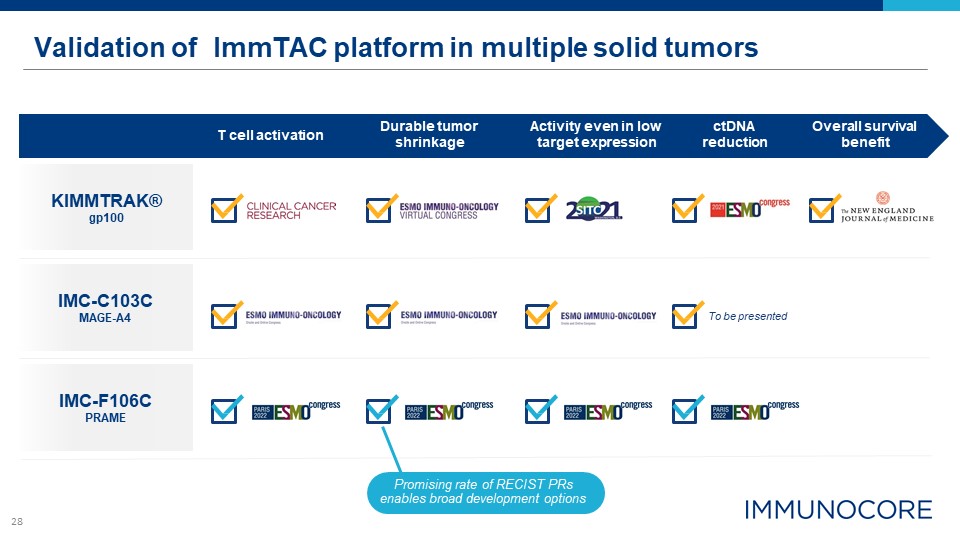
28 Validation of ImmTAC platform in multiple solid tumors KIMMTRAK® gp100 IMC-C103C MAGE-A4 T
cell activation Durable tumor shrinkage ctDNA reduction Overall survival benefit Activity even in low target expression To be presented IMC-F106C PRAME Promising rate of RECIST PRs enables broad development options

7 BRIAN DI DONATO Chief Financial Officer and Head of Strategy BAHIJA JALLAL, PhD Chief Executive
Officer DAVID BERMAN, MD, PhD Head of Research and Development Q&A Session MOHAMMED DAR, MD Chief Medical Officer OMID HAMID, MD The Angeles Clinic Chief, Translational Research and Immunotherapy and Co-Director, Melanoma
Therapeutics
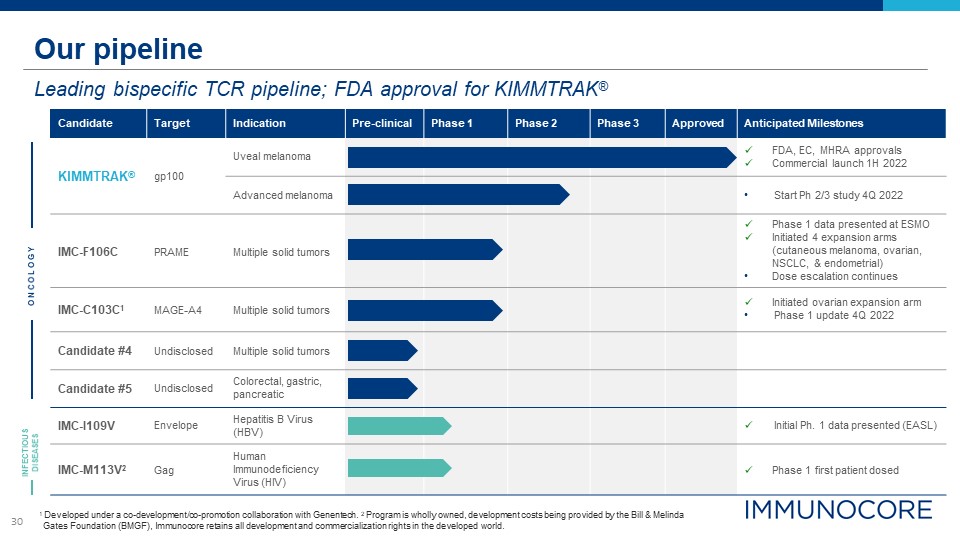
30 Our pipeline Leading bispecific TCR pipeline; FDA approval for KIMMTRAK® 1 Developed under a
co-development/co-promotion collaboration with Genentech. 2 Program is wholly owned, development costs being provided by the Bill & Melinda Gates Foundation (BMGF), Immunocore retains all development and commercialization rights in the
developed world. Candidate Target Indication Pre-clinical Phase 1 Phase 2 Phase 3 Approved Anticipated Milestones KIMMTRAK® gp100 Uveal melanoma FDA, EC, MHRA approvals Commercial launch 1H 2022 Advanced melanoma Start Ph 2/3
study 4Q 2022 IMC-F106C PRAME Multiple solid tumors Phase 1 data presented at ESMO Initiated 4 expansion arms (cutaneous melanoma, ovarian, NSCLC, & endometrial) Dose escalation continues IMC-C103C1 MAGE-A4 Multiple solid
tumors Initiated ovarian expansion arm Phase 1 update 4Q 2022 Candidate #4 Undisclosed Multiple solid tumors Candidate #5 Undisclosed Colorectal, gastric, pancreatic IMC-I109V Envelope Hepatitis B Virus (HBV) Initial Ph. 1 data
presented (EASL) IMC-M113V2 Gag Human Immunodeficiency Virus (HIV) Phase 1 first patient dosed O N CO L O G Y INFECTIOUS DISEASES
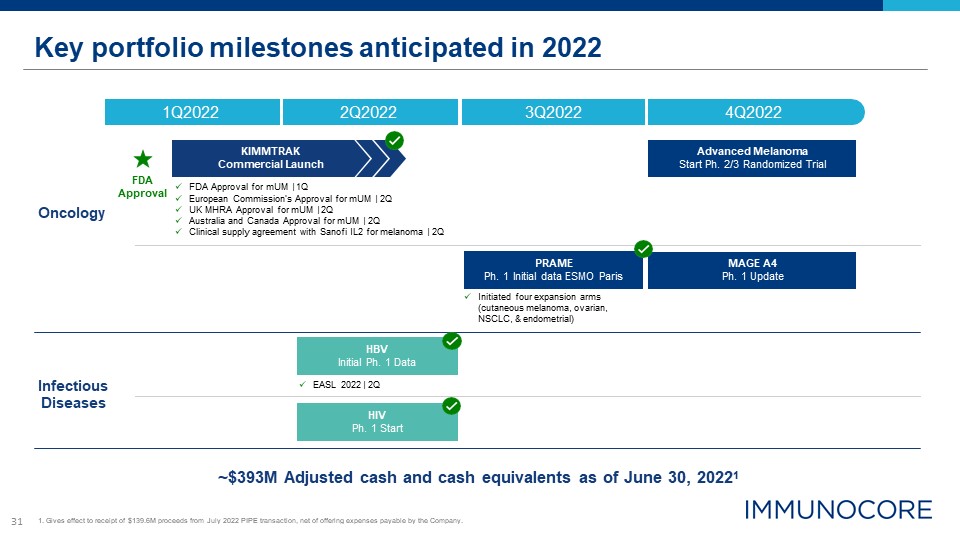
31 Key portfolio milestones anticipated in 2022 ~$393M Adjusted cash and cash equivalents as of June
30, 20221 Oncology Infectious Diseases FDA Approval 4Q2022 1Q2022 2Q2022 3Q2022 HIV Ph. 1 Start PRAME Ph. 1 Initial data ESMO Paris MAGE A4 Ph. 1 Update Advanced Melanoma Start Ph. 2/3 Randomized Trial KIMMTRAK Commercial
Launch FDA Approval for mUM | 1Q European Commission’s Approval for mUM | 2Q UK MHRA Approval for mUM | 2Q Australia and Canada Approval for mUM | 2Q Clinical supply agreement with Sanofi IL2 for melanoma | 2Q HBV Initial Ph. 1
Data EASL 2022 | 2Q 1. Gives effect to receipt of $139.6M proceeds from July 2022 PIPE transaction, net of offering expenses payable by the Company. Initiated four expansion arms (cutaneous melanoma, ovarian, NSCLC, & endometrial)